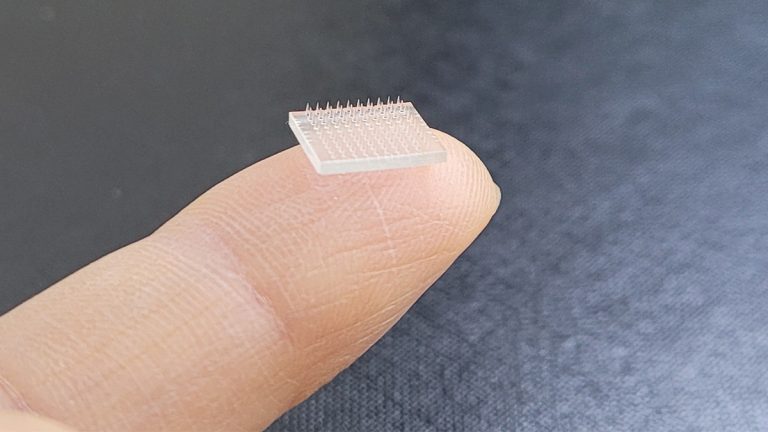
In an attempt to increase consumer demand for COVID-19 shots, researchers have designed a vaccine printer that can print COVID messenger RNA (mRNA) skin patches in bulk, producing as many as 100 patches in 48 hours. The microneedle patches, which contain mRNA molecules in lipid nanoparticles, could pave the way for unlimited distribution of the biologic as it would eliminate the need for doctors to administer the shots, dispose of needles, and refrigerate the product. The microneedle patches look like clear band-aids and would be self-applied to the skin, while the microscopic needles would break through the skin barrier and deliver the mRNA technology without the need for a medical worker to administer it.1
The microneedle patches measure approximately one-by-one-centimeter in length and contain a 10-by-10 grid of microneedles that vary in size to allow easier penetration of the skin. The microneedles have been dipped in a sugar solution of the COVID biologic. The microneedle patch developer explains that the skin has 100 to 1,000 times more migratory immune cells than muscle, thus allowing the skin patch to encounter significantly more immune cells than a traditional vaccine.2
This new technology has the potential to widely expand the purchase and use of mRNA biologics around the world. “With the possibility of scaling up vaccine manufacturing and improved stability at higher temperatures, vaccine printers can facilitate widespread access to mRNA vaccine,”3 said Joseph DeSimone, PhD, professor of translational medicine and chemical engineering at Stanford University,
3D Microneedle Vaccine Patch Creates Hyper Immune Response
The 3D printed vaccine patch first made news in 2021 when a study published in the Proceedings of the National Academy of Sciences showed that the microneedle patch created an immune response 10 times greater than a traditional needle injectable vaccine. The T-cell and antigen-specific antibody response was reportedly 50 times higher than seen with traditional vaccines.4
While microneedle patches had been considered as viable vaccine alternatives in the past, the use of 3D printing allows the microneedle patch to overcome hurdles that prevented microneedle patches to be used for different vaccine types. Using molds to make the patches in the past prevented the microneedles from remaining sharp over time, while the use of the 3D printer allows the microneedles to be consistently sharp enough to penetrate the skin.5
Unlike the current COVID mRNA biologics, the COVID mRNA microneedle patches do not need to be refrigerated and could be more easily shipped to developing countries. Research scientist Ana Jaklenec, PhD of the Massachusetts Institute of Technology suggests that the 3D printer itself could be sent to remote villages and refugee camps so the entire population could be swiftly vaccinated.6
The vaccine patch developers believe that vaccine rates can be increased through the use of this 3D technology. Dr. DeSimone said:
In developing this technology, we hope to set the foundation for even more rapid global development of vaccines, at lower doses, in a pain- and anxiety-free manner.7
Robert Langer, ScD, one of the microneedle patch study’s authors and a co-founder of Moderna’s Spikevax mRNA COVID biologic, said that he hoped the 3D printer and microneedle patch could be used for the “next COVID, or whatever crisis occurs.”
The 3D printed microneedle patches will not be available for at least two years.8
Live Virus Vaccines Being Tested for Skin Patch Delivery
Skin patches used as a vaccine delivery method are also being investigated for other biologics besides COVID shots. Micron Biomedical, Inc. of Atlanta, Georgia recently announced that its clinical trial of a microneedle patch for the live attenuated measles, mumps and rubella (MMR) vaccine completed Phase 1/2. The clinical trials have been conducted on babies nine months of age and older in India. Two more Phase 1/2 clinical trials are scheduled for the MMR vaccine patch.9
The MMR vaccine patches do not require cold storage or extra supplies such as needles and syringes like traditional vaccines, are easier to transport and application does not demand advanced medical training, which potentially will allow greater access to these vaccines around the world.10 11 Calls for investment funds for this technology have been made by Gavi, the Vaccine Alliance and its partners, the World Health Organization (WHO), UNICEF, the Bill & Melinda Gates Foundation, Path, CEPI and the U.S. Department of Defense (DoD) and the Biomedical Advanced Research and Development Authority (BARDA).12
Vaccine Skin Patches Raise Concerns
There are serious questions and concerns associated with this emerging technology. One question is whether recipients (or guardians of recipients) of a self-applied patch vaccine would receive sufficient information in order to make a fully informed decision. If the vaccine patches are purchased commercially, would a Vaccine Information Statement (VIS) be supplied as is required under the 1986 National Childhood Vaccine Injury Act (NCVIA) for government recommended vaccines and would other federal safety informing, recording and reporting provisions under that law be met prior to the purchase or use of the vaccine skin patch?
Another concern is that an employer could intimidate an employee into immediately putting a vaccine skin patch on rather than allowing the individual time to weigh the benefits and risks and make an appointment with a private doctor to discuss questions about the shot. It is much easier to slap a patch on someone’s arm without their consent than it is to inject someone with a needle.
Another risk of the live vaccine skin patch could be live vaccine virus shedding by recently vaccinated individuals. Similar to when a person has a viral infection and can transmit to others who come in contact with infected saliva, body fluids and skin lesions, a person who has recently received a live virus vaccine can shed the virus to people around them.
Post vaccination shedding can last from days to months depending on the vaccine and heath of the individual receiving the vaccine, with immune compromised people capable of shedding for longer periods of time.13 Someone who recently received the live attenuated influenza vaccine can shed the virus through respiratory secretions when breathing.
A 2018 study published in PNAS showed that individuals who received the live nasal flu shot shed six times more virus into the air compared to unvaccinated individuals.14 Whether a vaccine skin patch could have the potential to increase shedding because it is superficially injected into the top layer of the skin is an outstanding question.
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:1 Zhang J. New Vaccine Printers to Produce COVID-19 mRNA Shots on Demand. The Epoch Times Apr. 25, 2023.
2 Miller, K. A new approach to vaccinations: 3D printed patches. Scope 10k May. 12, 2022.
3 Zhang J. New Vaccine Printers to Produce COVID-19 mRNA Shots on Demand. The Epoch Times Apr. 25, 2023.
4 A 3D printed vaccine patch offers vaccination without a shot. University of North Carolina at Chapel Hill Sep. 23, 2021.
5 Ibid.
6 Scientists Develop Mobile Printer for mRNA Vaccine Patches. Science & Health Apr. 24, 2023.
7 A 3D printed vaccine patch offers vaccination without a shot. University of North Carolina at Chapel Hill Sep. 23, 2021.
8 New Mobile Printer Produces MRNA Vaccine Patches. Newsmax Apr. 26, 2023.
9 Geddes L. Needle-free vaccines could be available within five years, but investment is needed. Gavi, the Vaccine Alliance May 17, 2023.
10 Farmer B. Needle-free vaccines take the sting out of child immunization. The Telegraph May 19, 2023.
11 Geddes L. Needle-free vaccines could be available within five years, but investment is needed. Gavi, the Vaccine Alliance May 17, 2023.
12 Needle-free vaccine technology has potential to transform immunization. ET Health World May 19, 2023.
13 Fisher BL. The Emerging Risks of Live Virus & Virus Vectored Vaccines: Vaccine Strain Virus Infection, Shedding & Transmission. National Vaccine Information Center 2014.
14 Mercola J. Flu Vaccination Associated With Increased Viral Shedding. The Vaccine Reaction Aug. 25, 2020.