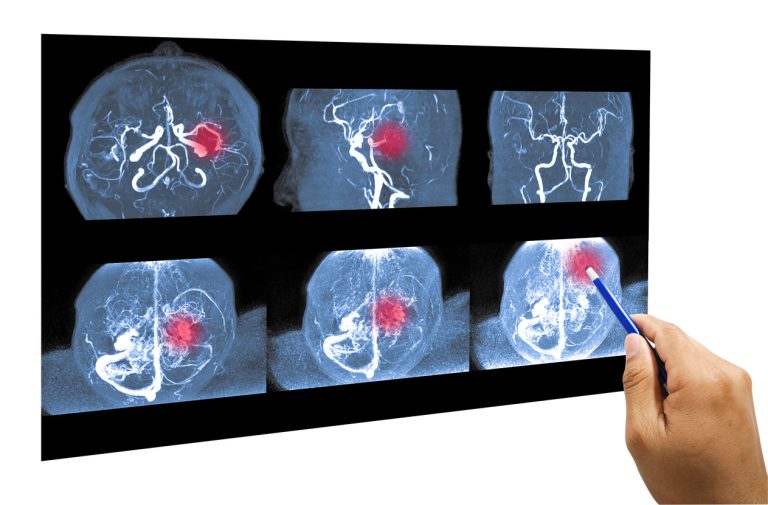
A new cohort study published in the British medical journal The BMJ on May 5, 2021 found increased rates of blood clots in veins, or “venous thromboembolism,” in people who received a first dose of AstraZeneca/Oxford University’s experimental COVID-19 vaccine (AZD1222) compared to blood clot rates in the broader population. Formerly known as ChAdOx1-S, AstraZeneca’s AZD1222 is a chimpanzee adenovirus vectored vaccine.1 2
The study involved 148,792 people in Denmark and 132,472 people in Norway who were given the AZD1222 vaccine between Feb. 9 to Mar. 11, 2021. The participants were all 18 to 65 years old and the median age for the study participants from Denmark and Norway was 45 and 44 years, respectively. Of the cohort from Denmark, 80 percent were women. Of the cohort from Norway, 78 percent were women.1 2
Using national health records from Denmark and Norway, study researchers were able to identify rates of arterial events, venous thromboembolism, thrombocytopenia/coagulation disorders and bleeding—”cardiovascular and haemostatic events”—that occurred within 28 days after vaccination and compare them to the general Danish and Norwegian populations.1 2
According to the results of the study, “59 venous thromboembolic events were observed in the vaccinated cohort compared with 30 expected based on the incidence rates in the general population.” This equates to an excess of 11 blood clot events per 100,000 vaccinations, including a higher-than-expected rate of rare brain blood clots (“cerebral venous thrombosis”)—2.5 cases per 100,000 vaccinations.1 2
37 Fatal Blood Clotting Cases After COVID-19 in Europe
The study was initiated following a report from the European Medicines Agency (EMA) in April that determined a “possibility of very rare cases of blood clots combined with low levels of blood platelets occurring within two weeks of vaccination” with AstraZeneca/Oxford’s AZD1222 vaccine. The EMA report was based on a review of 86 cases of blood clots in Europe through Mar. 22 following vaccination with AZD1222. Eighteen of the cases were fatal.3 4
Separately, a review by the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) identified blood clot cases in the country through March after AZD1222 vaccinations and 19 of the blood clot cases were fatal.4 5
AZD1222 Vaccine Banned in Denmark, Still Suspended in Norway
On Mar. 11, the government of Denmark suspended the use of AstraZeneca/Oxford University’s experimental COVID-19 vaccine, citing concerns about a possible link to cases of blood clotting. On Apr. 14, the Danish Health Authority (DHA) announced it would permanently halt the use of AstraZeneca/Oxford University’s coronavirus vaccine.6 The director general of the DHA, Søren Brostrøm, MD, said:
Based on the scientific findings, our overall assessment is there is a real risk of severe side effects associated with using the COVID-19 vaccine from AstraZeneca. We have, therefore, decided to remove the vaccine from our vaccination programme.6
Denmark is the first country to permanently ban the use of AZD1222. Norway also suspended AZD1222 on Mar. 11 over fears about blood clotting. The suspension will reportedly remain in effect until at least May 10, when an evaluation of the vaccine by the Norwegian government is due.7 8 9
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:1 Kelland K. Denmark, Norway study finds slightly raised blood clot rates after Astra COVID shot. Reuters May 5, 2021.
2 Pottegård A, et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. The BMJ May 5, 2021; 373: n1114.
3 Majeed Z. Denmark, Norway Study Finds Raised Blood Clot Rates After AstraZeneca Vaccine Shot. RepublicWorld.com May 6, 2021.
4 Holligan A. EU drug regulator: Unusual blood clot is ‘very rare AstraZeneca side effect’. BBC Apr. 7, 2021.
5 Triggle N. Covid: Under-30s offered alternative to Oxford-AstraZeneca jab. BBC Apr. 7, 2021.
6 Danish Health Authority. Denmark continues its vaccine rollout without the COVID-19 vaccine from AstraZeneca. Apr. 14, 2021.
7 Saigol L, Keown C. Denmark becomes first country to permanently stop use of AstraZeneca vaccine. MarketWatch Apr. 15, 2021.
8 RTT News. Norway Suspends AstraZeneca’s Covid-19 Vaccine Over Blood Clot Fears. Nasdaq Mar. 11, 2021.
9 NewsinEnglish.no. Latest Corona-related news in brief: May 6, 2021.