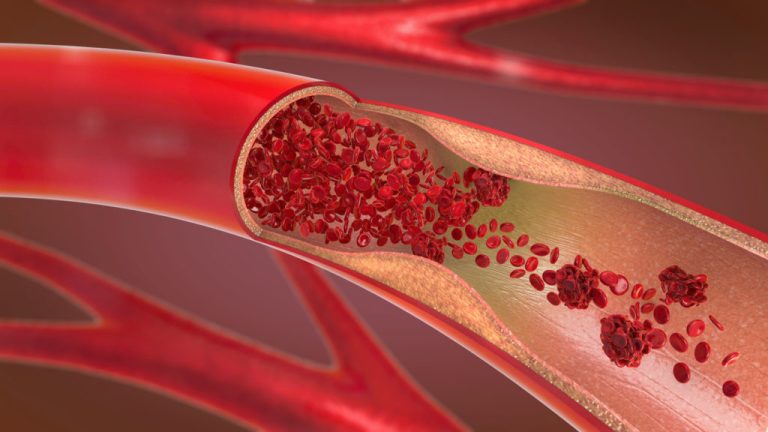
The European Medicines Agency (EMA) held an emergency meeting on Mar. 18, 2021 after many European countries suspended use of the Oxford/AstraZeneca experimental COVID-19 vaccine due to reports of severe side effects including blood clots, low platelet counts and death.1
The EMA conducted a preliminary investigation into potential serious side effects and pronounced the vaccine to be safe and effective with the benefits outweighing the risks, despite concerns that the vaccine has been linked to rare cases of blood clots in the vessels draining from the brain (CSVT) and low blood platelet counts, especially in recipients under the age of 55 years old. By early April, Germany, the Netherlands and Canada restricted use of the chimpanzee adenovirus vectored COVID-19 vaccine that was co-developed by Oxford University and AstraZeneca2 to people over age 55 or 60 due to the occurrence of pulmonary embolisms, brain hemorrhages and deaths in people between 25 and 60 years old, the majority of them women.3 4 5
After announcing that the Oxford/AstraZeneca vaccine was safe for distribution in mid-March, the EMA recommended updating the warning label for the experimental vaccine to better inform the public of the potential risks.6 7 8 Close to 20 countries, mostly in Europe, temporarily discontinued the use of the vaccine entirely or in batches after approximately 37 reports of cases of blood clots occurring after vaccination.9 10 11
Woman in Denmark Dies From Blood Clot After AstraZeneca Covid-19 Shot
Authorities in Denmark suspended all COVID-19 vaccinations with the Oxford/AstraZeneca brand after a 60-year old woman died from a blood clot after vaccination.12 Danish Health Minster, Magnus Heunicke, said the vaccination suspension was a temporary cautionary measure.13 And, Danish authorities clarified that “it has not been determined, at the time being, that there is a link between the vaccine and the blood clots.”14 Director of the National Board of Health in Denmark, Søren Brosttrøm said:
It is important to emphasize that we have not opted out of the AstraZeneca vaccine, but that we are putting it on hold. There is good evidence that the vaccine is both safe and effective. But both we and the Danish Medicines Agency have to react to reports of possible serious side effects, both from Denmark and other European countries. It shows that the monitoring system works.15
Other countries including Norway, Sweden Austria, Estonia, Latvia, Lithuania, Iceland, Luxenberg, Germany and Ireland also suspended the use of the Oxford/AstraZeneca COVID-19 vaccine in batches or altogether.16 17 18 Latvia, Lithuania, Luxenbourg and Bulgaria resumed use of the vaccine following the EMA’s conclusion it is safe,19 20 21 while Norway, Iceland and Denmark are continuing to review the data before proceeding with vaccination.22 Danish officials have said that once the vaccine is back in use, people will have the choice to refuse the Oxford/AstraZeneca vaccine in favor of other COVID-19 vaccines.23
Some Countries Restrict Oxford/Astra Zeneca Covid Vaccine to People Over Age 60
Finland’s Institute of Health and Welfare conducted their own investigation into two reports of blood clots after receiving the Oxford/AstraZeneca vaccine and have continued giving it to people over 65 years of age. Sweden has also returned to giving the vaccine, but only for persons aged 65 and older.24
Johan Carlsen, MD, head of the Swedish Public Health Agency said:
The vaccine is very useful for the elderly as many become seriously ill with COVID-19 every day… At the same time, we haven’t seen a risk of these rare and serious side effects in the elderly. That is the reason why we are canceling the break for people over the age of 65.25
Germany initially resumed use of the Oxford/AstraZeneca vaccine following the EMA conclusion it is safe, but then restricted its use to those over the age of 60 due to additional reports of blood clots occurring after vaccination. Germany’s independent vaccine committee, STICKO, said that their decision to restrict use of the vaccine to those over 65 was “based on the currently available data on the occurrence of rare but very severe thromboembolic side effects. This side effect occurred 4 to 16 days after vaccination, predominantly in people (under) 60 years of age.”26
The Netherlands, Iceland, Indonesia, Bulgaria and Thailand also chose to halt the use of the Oxford/AstraZeneca vaccine due to safety concerns following reports of blood clots post vaccination.27 28 29 30 The Netherlands, Iceland, Bulgaria and Thailand have resumed vaccination with the Astra Zeneca brand and Indonesia is using the vaccine with a warning for those with blood clotting disorders to not get the vaccine.31 32 33 34
Spain stopped vaccinating those between 55-65 years of age but has since continued vaccination.35 36
France announced that it would stop the use of the vaccine for 24 hours while Italy also announced it has curtailed the use of the vaccine as a precaution.37 After the preliminary findings by the EMA, both Italy and France began using the Oxford/Astra Zeneca vaccine again but with caveats. France is limiting its use to those aged 55 years or older and Italy is allowing citizens who refuse the Oxford/AstraZeneca vaccine to take another brand at a later time.38
Suspension of AstraZeneca Vaccine is the Latest in a Series of Controversies
The suspension and restrictions on using the Oxford/AstraZeneca COVID vaccine in Europe and Asia is the latest in series of controversies for the company. It began with a dispute over the Oxford/AstraZeneca’s COVID-19 vaccine clinical trial data, followed by a shortfall of vaccine supplies, and then doubts were raised about the effectiveness of the vaccine in the over 65 population due to the lack of data. Finally, the vaccine was suspended in many countries over concerns about the risk of developing a blood clot.39 40
Although most countries have resumed its use, some doubts remain about the vaccine. According to Sunaina Sinha Haldea, managing partner at Cebile Capital, AstraZeneca “seems to have a real PR problem on its hands in the U.S. and in Europe… public relations problem risks undermining confidence in the vaccine outside of the U.K.”41
Angela Merkel, the chancellor of Germany allegedly refused to get the vaccine because she does not, “belong to the group recommended for AstraZeneca.” The president of France, Emmanuel Macron, admitted that the Oxford/AstraZeneca COVID-19 vaccine was “quasi-ineffective” for people over the age of 65, one of the most vulnerable populations.
In Germany, people are not showing up for their appointments to receive the Oxford/AstraZeneca vaccine and only 107,000 doses have been given of the 737,000 doses received.42
AstraZeneca/Oxford Vaccine Supply Unused in Europe
Much of the Oxford/AstraZeneca COVID-19 vaccine supply has gone unused in Europe.43 44 According to the European Centre for Disease Prevention and Control, as of Mar. 11, France, Germany, Italy and Poland have used less than half of the doses of the vaccine that they have in stock.45 Europe falls far behind the U.S., Israel, the U.A.E., the U.K. and Chile in vaccinations per 100 people.46
European healthcare workers, including healthcare unions and a group of 3,000 doctors in Italy, have refused the Oxford/AstraZeneca COVID-19 vaccine due to efficacy and safety concerns. Some are insisting on receiving the Moderna/NIAID or Pfizer/BioNTech COVIC-19 vaccines, which they believe are more effective at reducing disease symptoms. Jerome Marty, MD, the president of a French Doctor’s union said, “Medical staff need the most effective vaccine. We need to keep the AstraZeneca vaccine for healthy and young people.” 47
When France began using the Oxford/AstraZeneca vaccine on Feb. 6, authorities reported seeing more side effects than they did with the other COVID-19 vaccines. The French medicines safety agency issued guidance to stagger the shots when given to front line workers to prevent disruption of operation due to the strong flu like side effects reported by 149 out of the 10,000 shots given.48
An AstraZeneca spokesperson defended its product, saying…
Regulators have clear and stringent efficacy and safety standards for the approval of any new medicine, and that includes COVID-19 vaccine by AstraZeneca. … The safety of the vaccine has been extensively studied in Phase III clinical trials and peer-reviewed data confirms the vaccine has been generally well tolerated.49
The World Health Organization (WHO) has maintained that there is no indication that the Oxford/AstraZeneca COVID-19 vaccine has caused the severe side effects including blood clots and death.50 AstraZeneca denies any connection to the risk of blood clots, noting…
Around 17 million people in the E.U. and U.K. have now received our vaccine, and the number of cases of blood clots reported in this group is lower than the hundreds of cases that would be expected among the general population.51
Emer Cooke, the head of the EMA stated that the safety committee “has come to a clear scientific conclusion. This is a safe and effective vaccine.” Despite assurances, the EMA has indicated it will continue to investigate the risk of blood clots with the Oxford/Astra Zeneca COVID-19 vaccine and has updated the vaccine’s warning label. 52 53
Researchers Find that the Oxford/AstraZeneca COVID-19 Vaccine Can Trigger Autoimmune Reaction and Blood Clots
Independent medical researchers in Germany and Norway discovered that an autoimmune reaction causing blood to clot in the brain could be triggered by the Oxford/AstraZeneca vaccine. Although the research has not yet been peer reviewed, the lead researcher in Norway, Pål André Holme, who identified the antibody that caused the blood clots due to the vaccine said, “Nothing but the vaccine can explain why these individuals had this immune response.”54
In Germany, researchers agreed with these findings and said that a blood test could diagnose CVST in people who have vaccine adverse reactions such as headaches, dizziness or impaired vision four days after they received the shot so that they can be treated with blood thinning medication and immunoglobulin. Robert Klamroth, MD, Deputy Chairman of the Society for Thrombosis and Hemostasis in Germany concluded:
We believe the most likely hypothesis is that this particular vaccine is causing a rare autoimmune reaction that triggers antibodies, which then interact with the platelets, but we don’t know why this is happening.55
Norway is holding off on restarting vaccination with the Oxford/AstraZeneca COVID-19 vaccine while Germany has resumed using it despite these findings.
In response, AstraZeneca relied on its earlier statement that CVST did not occur more frequently in those vaccinated than it would have in the general population. The EMA refuted that there was a causal link between the vaccine and CVST, but acknowledged the link is possible and further analysis warranted.
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:1 RT. European drugs regulator calls emergency vaccine summit after EU states press pause on AstraZeneca jab rollout. Mar. 15, 2021.
2 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
3 Jordans F. Germany to restrict AstraZeneca use in under-60s over clots. Associated Press/ABC News Mar. 30, 2021.
4 Duchamps EL. AstraZeneca Vaccine Suspended Again in Netherlands After Woman Who Received Jab Dies. New Tang Dynasty Apr. 2, 2021.
5 Miller A. Why Canada is suspending use of AstraZeneca vaccine in people under 55. CBC News Mar. 29, 2021.
6 Schnirring L. European regulators say AstraZeneca vaccine safe, but can’t rule out rare events. Center for Infectious Disease Research and Policy Mar. 18, 2021.
7 Amaro S. European nations resume use of AstraZeneca Covid vaccine after regulator signs off. CNBC Mar. 18, 2021.
8 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
9 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
10 Jordans F. Germany to restrict AstraZeneca use in under-60s over clots. Associated Press/ABC News Mar. 30, 2021.
11 Covid-19: EU states to resume AstraZeneca vaccine rollout. BBC News Mar. 19, 2021.
12 Covid-19: Several European countries suspend AstraZeneca vaccinations over blood clot fears. France24 Mar. 11, 2021.
13 Mortensen A, Schams Elwazer S, Siad A. More European nations pause AstraZeneca vaccine use as blood clot reports investigated. CNN Mar. 11, 2021.
14 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
15 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
16 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
17 Dean, Grace, Schuster-Bruce, Dr. Catherine. Sweden joins Germany, France, and 15 other countries in suspending AstraZeneca’s vaccine over possible side effects. Business Insider Mar. 16, 2021.
18 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
19 European Countries To Restart Using AstraZeneca Vaccine: Report. FIT Mar. 19, 2021.
20 Jordans F. Germany to restrict AstraZeneca use in under-60s over clots. Associated Press/ABC News Mar. 30, 2021.
21 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
22 Factbox: Countries resume use of AstraZeneca vaccine, while some lose confidence. Reuters Mar. 22, 2021.
23 Olsen, Jan. Denmark prolongs suspension of AstraZeneca COVID-19 vaccine. AP News Mar. 25, 2021.
24 Ibid.
25 European Countries To Restart Using AstraZeneca Vaccine: Report. FIT Mar. 19, 2021.
26 Ellyat H. Germany suspends use of AstraZeneca’s Covid shot for the under-60s, dealing another blow to drugmaker. CNBC Mar. 31, 2021.
27 Thepgumpanat P, Rinke A. Thailand clears AstraZeneca use as potential side-effects divide Europe. Nasdaq Mar. 15, 2021.
28 Lardieri A. More Countries Suspend AstraZeneca Coronavirus Vaccine Over Health Concerns. U.S. News & World Report Mar. 12, 2021.
29 Factbox: Countries resume use of AstraZeneca vaccine, while some lose confidence. Reuters Mar. 22, 2021.
30 Jordans F. Germany to restrict AstraZeneca use in under-60s over clots. Associated Press/ABC News Mar. 30, 2021.
31 Iceland resumes use of AstraZeneca’s COVID-19 vaccine. Reuters Mar. 25, 2021.
32 Bulgaria to resume vaccinations with AstraZeneca’s shots. Yahoo! News Mar. 18, 2021.
33 Thailand resumes use of AstraZeneca vaccine. Kydo News Mar. 16, 2021.
34 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
35 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
36 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
37 Mortensen A, Schams Elwazer S, Siad A. More European nations pause AstraZeneca vaccine use as blood clot reports investigated. CNN Mar. 11, 2021.
38 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
39 Sagonowsky E. Seven European countries clamp down on AstraZeneca COVID-19 vaccine as safety worries threaten rollout. Fierce Pharma Mar. 11, 2021.
40 Ellyatt H. Data, doubts and disputes: A timeline of AstraZeneca’s Covid vaccine problems. CNBC Mar. 25, 2021.
41 Ibid.
42 Ring S, Fourcade M. EU Regulator Deems Astra Safe, But Recommends Warning Label. Bloomberg Mar. 18, 2021.
43 Ibid.
44 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
45 Ibid.
46 Ibid.
47 McEvoy J. European Healthcare Workers Are Refusing AstraZeneca Vaccine Over Efficacy Concerns. Forbes Feb. 22, 2021.
48 Pailliez C, Ahlander J. AstraZeneca vaccine faces resistance in Europe after health workers suffer side-effects. Reuters Feb. 18, 2021.
49 BBC. Covid: What is the Oxford-AstraZeneca vaccine? Mar. 22, 2021.
50 Factbox: Countries resume use of AstraZeneca vaccine, while some lose confidence. Reuters Mar. 22, 2021.
51 Mortensen A, Schams Elwazer S, Siad A. More European nations pause AstraZeneca vaccine use as blood clot reports investigated. CNN Mar. 11, 2021.
52 Cheng M, Jordans F. EU agency: AstraZeneca vaccine safe, will add clot warning. ABC News Mar. 18, 2021.
53 Jordans F. Germany to restrict AstraZeneca use in under-60s over clots. Associated Press/ABC News Mar. 30, 2021.
54 Pancevski B. Scientists Say They Found Cause of Rare Blood Clotting Linked to AstraZeneca Vaccine. The Wall Street Journal Mar. 19, 2021.
55 Ibid.













3 Responses
Have any of these people been screened for blot clot tendencies, such as the Leiden V factor? “Factor V Leiden (FAK-tur five LIDE-n) is a mutation of one of the clotting factors in the blood. This mutation can increase your chance of developing abnormal blood clots, most commonly in your legs or lungs”
Folks should be asking questions like this.
Apr 1, 2021 Why Autism is more prevalent among boys in today’s world
Autism is increasing in huge numbers all over the world. The United Nations recognised April 2 as World Autism Awareness Day urging all its members to take up the social cause and raise awareness in the society.
https://youtu.be/B2K7o6Bdb4s
I am not willing to get any vaccine as of April 2021.
They do too little for me to take chances.
I am 82 as of May and will take my chances treating to prevent infection.