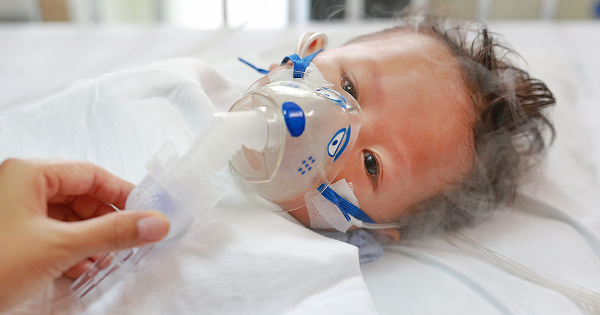
On Dec. 11, 2024, the U.S. Food and Drug Administration (FDA) announced that clinical trials for Moderna’s pediatric mRNA RSV vaccines have been paused due to safety concerns. The recent phase 1 trials of two experimental respiratory syncytial virus (RSV) vaccines for infants not only failed to provide adequate protection but may have worsened respiratory symptoms in some babies who contracted RSV or other respiratory viruses. The pause followed reports of five severe to very severe lower respiratory tract infections caused by RSV among the 40 infants who received the vaccine, compared to just one case in the group of 20 infants who received a placebo. Five RSV vaccinated infants were hospitalized, with one requiring mechanical ventilation.1 2
RSV is a common virus that infects the lungs and respiratory tract and is spread easily through coughing, sneezing, or contact with contaminated surfaces. While it typically causes mild illness in healthy individuals, infants, and older adults, those with weakened immune systems face a higher risk of severe complications or hospitalization.1
Vaccine-Associated Enhanced Respiratory Disease a “Significant Concern” for RSV Vaccine Development
The FDA’s briefing stated that the trial’s findings “has uncertain implications for the ongoing and future pediatric development of other non-live attenuated [inactivated] RSV vaccines.” The document also emphasizes that the potential for vaccine-associated enhanced respiratory disease (VAERD) remains a significant concern for RSV vaccine development in infants and young children. The briefing highlights that historical failures, such as the 1960s trial where two children died, underscore the need for cautious evaluation of immune responses to avoid exacerbating illness.2
When Protection Failed: The Fatal RSV Vaccine Trial of the 1960s
The road to RSV protection has been long and fraught with setbacks, often raising more questions than solutions for scientists. One of the most tragic moments occurred in the 1960s during clinical trials for an RSV vaccine, where 80 percent of the children who received the shot were hospitalized after contracting the virus naturally. The inactivated virus used in the vaccine not only failed to protect but worsened the disease, resulting in the deaths of two children. The trial was immediately halted, and researchers were forced to rethink their strategy and seek new solutions.3
It took nearly 60 more years before the FDA would approve the first RSV vaccine—GlaxoSmithKline’s Arexvy in May 2023—for use in adults aged 60 and older. Moderna’s mRNA-1345, marketed as mRESVIA, was later approved by the FDA in May 2024. It is one of three RSV vaccines—alongside GSK’s Arexvy and Pfizer’s Abrysvo—licensed for adults, with Abrysvo also approved for pregnant women. Still, reports of the nervous system disorder Guillain-Barré syndrome (GBS)—a common complication associated with vaccines, particularly the flu and tetanus vaccines—were “more common than expected” in the older adult population who received the new vaccine for RSV, according to the U.S. Centers for Disease Control and Prevention (CDC).4
Anti-Vaccine Community Might “Leverage” RSV Trial Failures
Some people have shared concerns that those with “anti-vaccine agendas“ may exploit the findings of the now-halted trial. “We want this vaccine field to move forward and this is a great thing for them to jump on and say, ‘Oh, this is so toxic, scary, we can’t do this,” pediatrician Coleen Cunningham, MD said in an interview. “I think that would be a mistake.”1
Barney Graham, PhD, the virologist and immunologist behind the newer RSV development strategy, takes a more balanced, risk-averse approach to the trial outcomes. “We need to remain humble about what we actually know,” Dr. Graham says. “I’m thankful that problems didn’t occur in virtually every child, as happened in the 1960s. My main concern is that we invest in basic research to help us move on from this point and find a solution for children between six months and five years of age.”1
The hospitalizations from the clinical trial have raised broader concerns about the safety of mRNA-based vaccines in young children. Moderna will continue to monitor trial participants and gather data, but the trial for children under 2 is not expected to resume. The timeline for restarting is uncertain until safety evaluations are finalized.1
If you would like to receive an e-mail notice of the most recent articles published in The Vaccine Reaction each week, click here.
Click here to view References:1 Greenfield B. Moderna pauses its RSV vaccine trial for children after 5 infants are hospitalized in latest challenge of preventing disease. Yahoo News Dec. 16, 2024.
2 U.S. Food and Drug Administration. FDA briefing document: Vaccines and Related Biological Products Advisory Committee meeting.
3 Harding A. Research shows why 1960s RSV shot sickened children. Reuters Dec. 3, 2008.
4 Hobley N. Guillain-Barré Syndrome ‘More Common Than Expected’ in Early RSV Vaccine Trial. The Vaccine Reaction June 25, 2024.












3 Responses
Don’t believe your lying eyes. Humble yourself and support growing big pharma profits. Get your vaccines. One ponders if the parents took the children home immediately, and did not let them stay or go back to the hospital, if the children would have been in at any risk in the first place. FDA to the pharmasuetical company; ‘We had an agreement the children would not die right away. Go reformulate and we’ll re approve asap.’
There is a special place in hell for people who experiment on innocent little babies.
In other news, the one child whom received the placebo, but still fell ill, likely came in contact with the virus, by way of the other infants in the same complex whom received the shot.