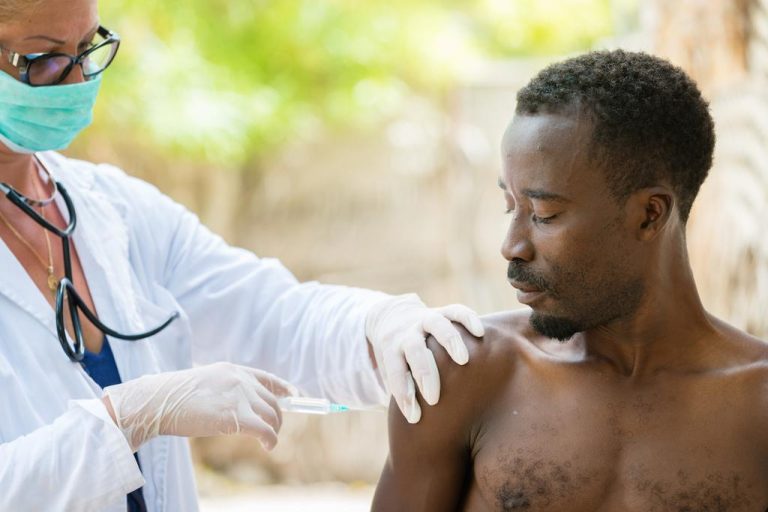
Kenya has become the third African country, along with Ghana and Malawi, to launch a pilot program for the world’s first malaria vaccine, Mosquirix, which has been developed by GlaxoSmithKline (GSK). The vaccine is available to children from six months of age in specific locations in Kenya due to its phased pilot introduction.1 2
Malaria is a mosquito-borne disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes.3 It is preventable and curable. Symptoms of malaria include fever, headache and chills.
The African region carries a disproportionately high share of the global malaria burden. According to the U.S. Centers for Disease Control and Prevention (CDC), malaria occurs mostly in poor tropical areas of the world and Africa is affected the most due to “scarce resources and socio-economic instability have hindered efficient malaria control activities.”3 4
The pilot program is being coordinated by the World Health Organization (WHO) in collaboration with Kenya’s Ministry of Health and international partners, including global health non-profit PATH of Seattle, Washington and the United Kingdom’s GSK which has donated 10 million vaccine doses for the program.1
The malaria vaccine will be administered to children in four doses: three doses between six and nine months of age and the fourth dose at two years old. The goal is to vaccinate more than 120,000 children per year in Kenya across the selected introduction areas including Homa Bay, Kisumu, Migori, Siaya, Busia, Bungoma, Vihiga and Kakamega counties. Within the eight counties, some sub-counties will introduce the vaccine into vaccine schedules, while others are expected to introduce the vaccine later.2 5
According to a document on the website of the European Medicines Agency (EMA), the identified risks of the vaccine include febrile convulsions and other the potential complications include meningitis, hypersensitivity (including anaphylaxis), immune related disorders, rebound effects and cerebral malaria.6
References:
1 World Health Organization. Malaria vaccine launched in Kenya: Kenya joins Ghana and Malawi to roll out landmark vaccine in pilot introduction. WHO Regional Office for Africa Sept. 13, 2019.
2 Ree V. Kenya launches phased malaria vaccine pilot programme. European Pharmaceutical Review Sept. 16, 2019.
3 WHO. Malaria. WHO.int Mar. 27, 2019.
4 Centers for Disease Control and Prevention. Malaria’s Impact Worldwide. CDC.gov Jan. 4, 2019.
5 Saya M. WHO to use key data from malaria vaccine pilots for policy making. The Star Sept. 16, 2019.
6 European Medicines Agency. Summary of risk management plan for Mosquirix. EMA.Europa.eu July 30, 2019.












10 Responses
Now that’s scary. Three injections between 6 and 9 months of age, when a child’s immune system is still undeveloped. There’s already a viable alternative that conventional Western medicine refuses to consider–a homeopathic remedy made from Cinchona bark. It has been used successfully for two centuries to treat malaria, and is better than anything Western medicine has come up with. And the best part? Truly safe and effective.
This is very scary territory to be in – they feel the urgency of solving over ½ millions cases of malaria, they have obviously 1-2 big “payers” and they have the components required to make it. (my guess is one is the Gates Foundation)
They don’t have any safety studies or any time to develop them. The significance of safety studies may not be obvious – they tell you how any children die from the vaccine and what they die from. I realize that’s an old concept based on the idea of “informed consent” – that’s where you tell people in advance what the risks are in what you’re about to do to them – really that concept has been dead for quite some time but it’s convenient to make the population think they still have a choices as we wait for the courts in each of the U.S. states to close every loophole left. This is typical of the “tobacco logic” run rampant.
Just look at this one very scary comment from their published info (like a package insert) https://www.ema.europa.eu/en/documents/medicine-outside-eu/mosquirix-product-information_.pdf – page 4 is saying “Meningitis – In clinical studies, meningitis (any aetiology) has been reported more frequently in the group vaccinated with three doses of Mosquirix up to 20 months post dose 1 (27 cases out of 11,439 vaccinees) compared with the control group (4 cases out of 6,096 vaccinees). A causal relationship to the vaccine has not been established.” In case you can’t decipher this, the vaccine caused a 33X increase in Meningitis. That’s about 3300% – I’m not a scientist – just a guy with a calculator who can read research reports. And they stated “A causal relationships to the vaccine has not been established” because they was not enough peer-reviewed testing to determined anything.
Sounds like another disaster?
Malaria is indeed the curse of central Africa. The most common reason people are hospitalized is malaria. There is no known effective prevention or long -term cure known to residents of Zambia or neighboring countries. If cinchona bark is effective I would like to know more about it
Malaria is indeed the curse of central Africa. The most common reason people are hospitalized is malaria. There is no known effective prevention or long -term cure known to residents of Zambia or neighboring countries. If cinchona bark is effective I would like to know more about it.
Quinine, derived from cinchona, is not very effective and does little to eradicate the recurring bouts of fever that afflict malaria victims repeatedly for years after being infected. An effective vacation without bad side effects would be a blessing.
I pray for these babies.
Get ready to have millions of African children to be permanently maimed/injured and/or die — they are effectively going to wipe out a whole generation of children….
Peter Aabys group – RTS,S/AS01 malaria vaccination with and without a booster was associated with vaccine efficacies (VE) against clinical malaria of 18–36%. Modelling has estimated that this vaccine would reduce mortality by 6–11 deaths per 1000 vaccinees.
However, no significant VE against all-cause mortality was identified. In fact, although not statistically significant, RTS,S/AS01 was associated with a 24% (95% CI −3% to 58%) increase in overall mortality, corresponding to four additional deaths per 1000 vaccinees. After the initial reports, a significant increase in mortality of 50% (1–124%) from 14 months to the end of the study was reported in the RTS,S/AS01 groups with and without a booster.
One explanation could be that early RTS,S/AS01 vaccination prevents the normal development of malaria immunity. In support of this, older RTS-vaccinated children who received no booster had a significant increase in severe malaria from month 21 to the end of the study. Furthermore, fatality from severe malaria was noted in 21 (3·2%) of 648 patients in the RTS,S/AS01 groups and six (1·6%) of 388 patients in the control group (RR 2·10, 0·85–5·15)
The higher than expected overall mortality could also be due to non-specific effects of vaccines. Live vaccines might increase general resistance leading to lower overall mortality, but some inactivated vaccines increase susceptibility to other infections leading to higher overall mortality. The inactivated RTS,S/AS01 vaccine could have negative non-specific effects counterbalancing the beneficial specific effects. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)00693-5/fulltext
Peter Aabys paper on non specific effects: https://www.ncbi.nlm.nih.gov/pubmed/31449870
RTS,S/AS01 malaria vaccination in young infants with and without a booster was associated with vaccine efficacies of 18–36%. The Lancet supports a large-scale vaccine roll-out after publication of these final results from a multicentre phase 3 study. We would argue for a more cautionary approach and call for additional studies before considering the inclusion of this vaccine in the routine Expanded Programme for Immunizations schedule.
First, the vaccine provides only modest and short-lived protection. Cost-effectiveness needs to be established to justify large-scale roll-out of a partially effective malaria vaccine in endemic countries. Second, safety of the vaccine is an issue with an unexplained excess of meningitis cases reported in the RTS,S group.Third, data cannot confirm yet if and how the inactivated RTS,S/AS01 will affect overall child mortality. Although live vaccines overall seem to be beneficial, inactivated vaccines have been associated with negative non-specific effects, including increased child mortality. Finally, malaria interventions in young children might lead to rebound morbidity and mortality in older age groups. Long-term follow-up studies were undertaken before the insecticide-treated mosquito net intervention was recommended for large-scale roll-out in endemic areas. This process should also apply to malaria vaccines. A malaria vaccine would be an important complement to existing preventive and treatment measures, but well-designed long-term studies are warranted before a decision is made to include a malaria vaccine in the Expanded Programme for Immunizations. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(15)00694-7/fulltext
final results of a phase 3, individually randomised, controlled trial https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5626001/
what about sodium chlorite plus activator/mms – asserted to be demonstrated in a video of a field study conducted by the red cross – which it appears they have since distanced themselves from?
Or, for that matter, any of the readily available natural parasite medications.
https://jimhumble.co/videos/#red_cross_cured_malaria_with_mms
Z
I guess I may be naive – but what about houses with windows and screens? What about storm drainage and a good sewer system? Are all these things in place in the malaria- stricken areas?