Two Elderly Men Die Hours After Getting COVID-19 Vaccines in Israel

A 75-year-old man suffered a heart attack and died two hours after receiving the first dose of Pfizer/BioNTech’s experimental messenger RNA (mRNA) BNT162b2 vaccine for COVID-19 at a local health clinic in Israel.1 2 3 The elderly man, who was reported to have cancer and a history of heart attacks, was administered the vaccine at […]
So What Happens if You Get Injured by a COVID-19 Vaccine?

Opinion | The experimental COVID-19 vaccines are being released first to a designated group of health care workers and residents of senior living facilities. As more of the vaccines becomes available, people at high-risk to the SARS-CoV-2 virus due to their medical conditions will be given an opportunity to get vaccines. Finally, the general public […]
Patient in Georgia, Health Worker in New York Suffer Anaphylaxis After Getting Pfizer/BioNTech COVID-19 Vaccine
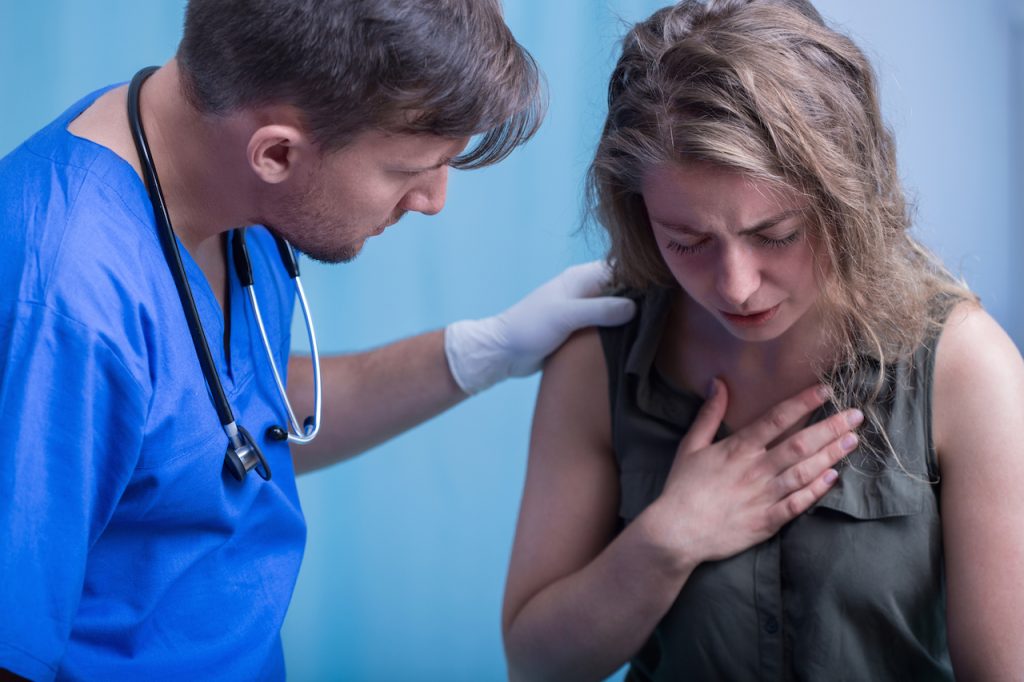
On Dec. 22, 2020, a patient at Decatur Morgan Hospital in Decatur, Alabama suffered a severe allergic reaction called anaphylaxis just minutes after receiving Pfizer/BioNTech’s experimental messenger RNA (mRNA) BNT162b2 vaccine for COVID-19.1 2 3 In a press release, the Alabama Department of Public Health (ADPH) stated the patient, who reportedly had a history of […]
Over 3,000 “Health Impact Events” After COVID-19 mRNA Vaccinations
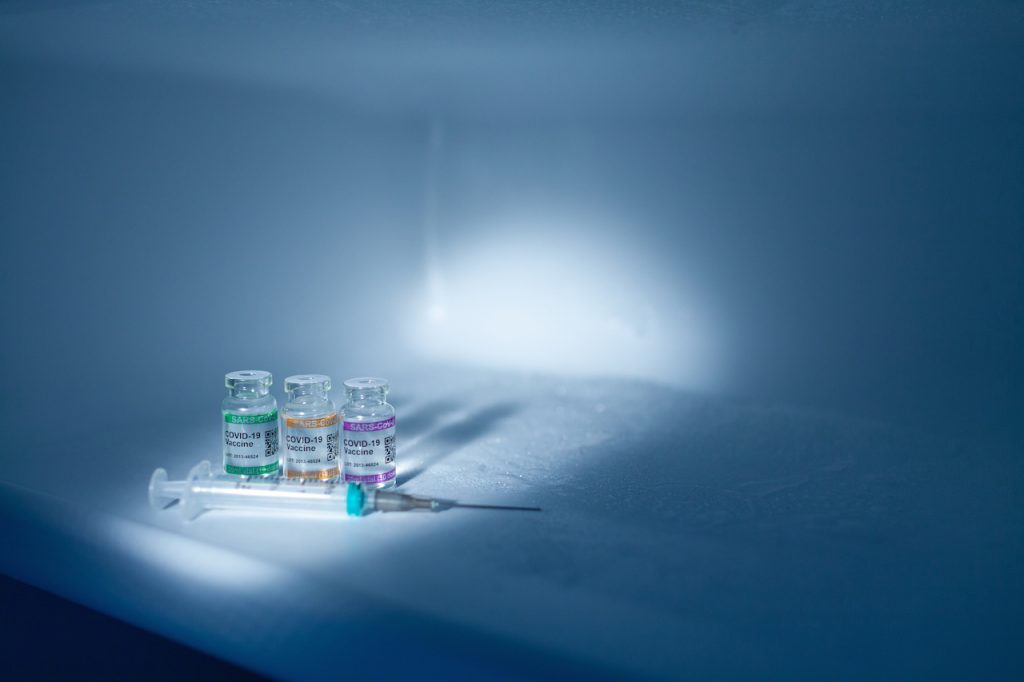
Between Dec. 11 and 18, 2020, the U.S. Food and Drug Administration (FDA) granted Pfizer/BioNTech and Moderna pharmaceutical companies an Emergency Use Authorization (EUA)1 to distribute COVID-19 vaccines using messenger RNA (mRNA) technology that to date has not been licensed for use in humans.2 3 4 5 Although the Advisory Committee on Immunization Practices (ACIP) […]
Peruvian Man Suffers Guillain-Barré-like Symptoms After Receiving Sinopharm COVID-19 Vaccine

On Dec. 11, 2020, Peru’s National Institute of Health (INS) said it was temporarily suspending the Phase 1 clinical trial of an experimental COVID-19 vaccine developed by Sinopharm Group Co. Ltd. of Shanghai, China after one of the participants in the trial who got the vaccine showed difficulty in moving his arms and legs—symptoms of […]
Russians Warned to Abstain from Drinking Alcohol After Getting Sputnik V COVID-19 Vaccine

Story Highlights Health officials in Russia tell Russians to avoid drinking alcohol two weeks prior to and 42 days after receiving the emergency use Sputnik V COVID-19 vaccine The Sputnik V manufacturer’s alcohol warning contradicts That of Russian health officials. The manufacturer recommends refraining from alcohol three days after each of the two doses of […]
