Influenza Vaccines Only 42 Percent Effective in Adults This Year

The influenza vaccines currently being administered in the United States are estimated by the U.S. Centers for Disease Control and Prevention (CDC) to be 42 percent effective in adults. This is an interim estimate for the 2023-2024 “flu season” published in the agency’s Morbidity and Mortality Weekly Report (MMWR) on Feb. 29, 2024.1 2 3 […]
Pharma Developing Bird Flu Vaccine for Humans As Animals Get Sick
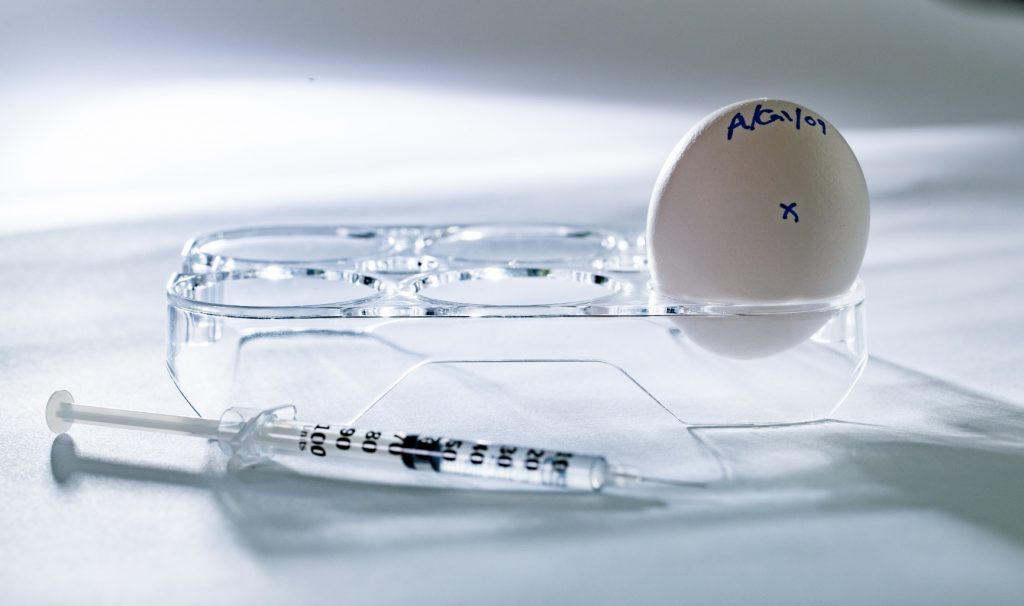
Pharmaceutical companies GSK (formerly known as GlaxoSmithKline), Moderna and CSL Seqirus announced that they are developing and ready to test human vaccines against avian influenza H5N1 (also known as bird flu) as a precautionary measure to prepare for what they believe may be a future pandemic.1 Now that certain strains of H5N1 have infected not […]
CDC Finds Glyphosate in Urine of Majority of Children in U.S.

A recent analysis by the U.S. Centers for Disease Control and Prevention (CDC) revealed that urine samples of approximately 87 percent of 650 children living in the United States had detectable levels of the weed killer pesticide glyphosate.1 What is Glyphosate? Glyphosate is the most widely used herbicide in the U.S. in the agricultural industry […]
Flu Shot Deaths in South Korea Said to Be “Coincidental”

Story Highlights More than 80 people have died in South Korea following influenza vaccination. After an investigation into the safety of the vaccines, South Korean health authorities ruled the deaths as “coincidental.” The public health agency in South Korea has decided to continue and promote its free influenza vaccine program despite public anxiety relating to […]
ACIP Updates Influenza Vaccine Guidelines for Seniors

The Advisory Committee on Immunization Practices (ACIP) for the U.S. Centers for Disease Control and Prevention (CDC) has issued new updates for influenza vaccines for 2020–2021 “flu season,” primarily for Fluzone and Fluad vaccines. In November 2019, U.S. Food and Drug Administration (FDA) licensed inactivated injectable Fluzone High-Dose Quadrivalent (HD-IIV4) for use in persons aged […]
Virginia Legislature Gives CDC, Board of Health Power to Mandate Vaccines for School Children

In the 2020 Legislative session, the Virginia Legislature passed H.B.1090, a bill that amended a law requiring children attending public and private schools in the state to receive vaccines (§ 32.1-46. Immunization of patients against certain diseases).1 Virginia Delegate Patrick Hope (D-Arlington) was the lead sponsor of H.B. 1090 introduced on Jan. 8, 2020, which […]

