Safety and Effectiveness of New COVID Bivalent Boosters Based on a Study of Eight Mice?

With no safety and efficacy evidence from human clinical trials, on Aug. 31, 2022, the U.S. Food and Drug Administration (FDA) amended the Emergency Use Authorizations (EUAs) for Pfizer/BioNTech’s Comirnaty and Moderna/NIAID’s Spikevax messenger RNA (mRNA) COVID-19 biologics to distribute “bivalent formulations” (two-in-one) of the shots to use as a “single booster dose.”1 2 The […]
A Gratitude Script for the COVID Vaccinated But Infected

“I would like to let you know that I have tested positive for #COVID19. I am thankful to have received four doses of the Pfizer-BioNTech vaccine, and I am feeling well while experiencing very mild symptoms. I am isolating and have started a course of Paxlovid.” These were the words of Pfizer CEO Albert Bourla […]
Nuvaxovid Offered as Alternative to mRNA Shots

For a virus (SARS-CoV-2) that many scientists and public health officials have maintained originated in a bat and accidently crossed over into humans, leading to the COVID-19 pandemic, it is odd that there does not appear to be much concern about producing vaccines that use genetic material from animals. You would think that this would […]
VAERS Data Reveal Serious Adverse Event Reports in Babies After mRNA COVID Shots
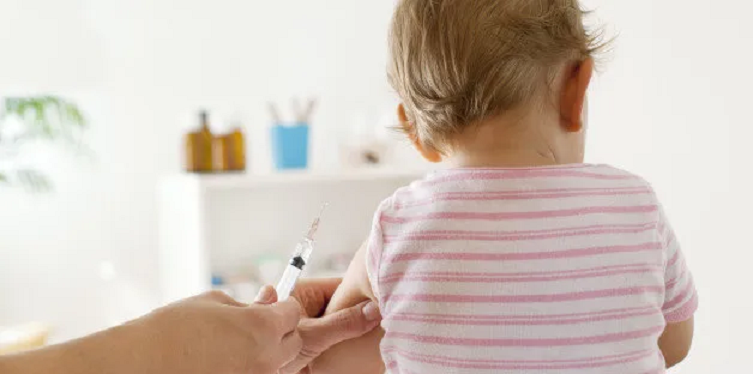
On June 15, 2022, the Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the U.S. Food and Drug Administration (FDA) advised the FDA to grant an Emergency Use Authorization (EUA) for Moderna/NIAID and Pfizer/BioNTech’s mRNA COVID-19 biologics be given to infants and children under age five and as young as six months old. With […]
FDA Grants EUA for Novavax’s COVID Vaccine
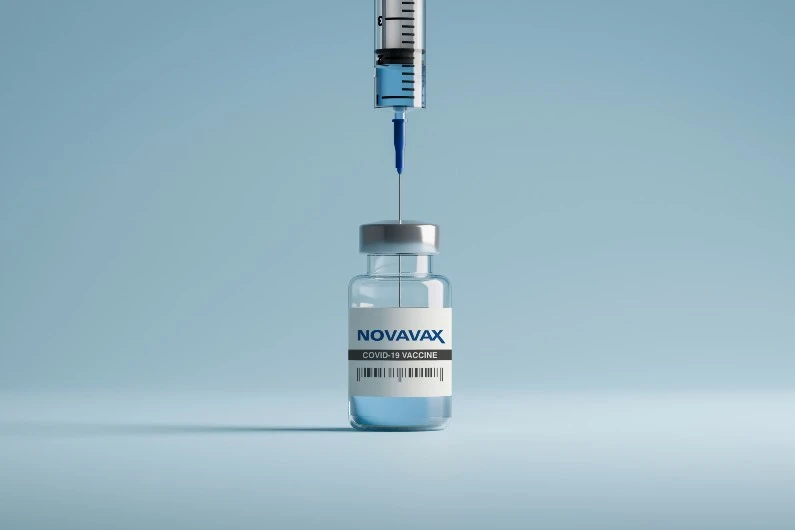
Opinion | On July 13, 2022, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) to Novavax to distribute its experimental two-dose NVX-CoV2373 (also known as “Nuvaxovid” and “Covovax”) COVID-19 vaccine for use by adults. The decision was expected, given the favorable recommendation by the FDA’s Vaccines and Related Biological Products Advisory […]
‘COVID-19 Rebound’ May Be Worse Than Initial COVID Illness
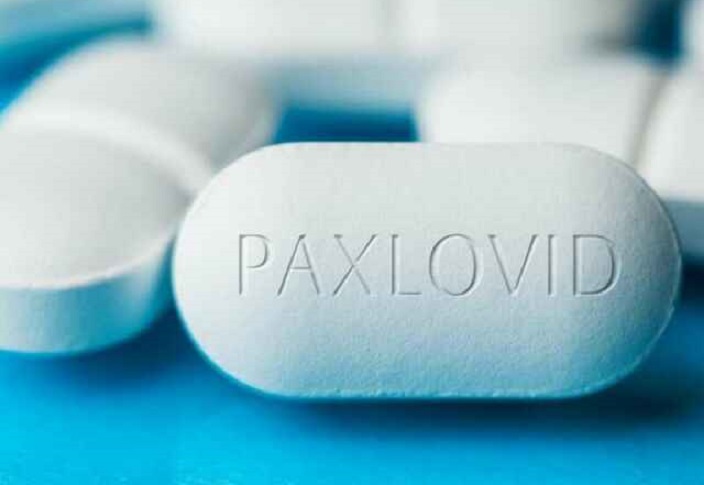
On May 24, 2022, the U.S. Centers for Disease Control and Prevention (CDC) issued a health advisory to health care providers, public health departments and the general public regarding Pfizer’s antiviral drug Paxlovid, designed to treat symptoms of COVID-19. The advisory warns that some people who take Paxlovid may experience a recurrence of COVID symptoms […]
