Three German Scientists Call for Immediate Halt on All mRNA Biologicals, Including COVID Shots
German authors of a new peer-reviewed study called for an “immediate halt of all mRNA biologicals,” ” including COVID-19 shots, until questions about residual DNA that well exceeds regulatory limits can be answered. The study, published on Dec. 3, 2024 in the Public Health Policy Journal, underscores concerns about the mRNA technology that includes the […]
Man Suffers Painful Facial Nerve Damage After Pfizer’s mRNA COVID-19 Booster Shot

A 54-year-old man from the Omagh, Northern Ireland suffered a severe adverse reaction just days after receiving the Pfizer/BioNTech’s Comirnaty mRNA (messenger ribonucleic acid) COVID-19 booster shot in December 2021. Larry Lowe, a former college lecturer, musician and runner, said he developed numbness on the right side of his face and began to experience severe […]
Influenza-COVID Combo Vaccine Trial Halted Over Safety Concerns

The U.S. Food and Drug Administration (FDA) has put a hold on Novavax’s COVID-influenza combination vaccine and standalone influenza vaccines being tested in clinical trials after a participant reported nerve damage following receipt of the experimental vaccine in January 2024.1 Reported symptoms were diagnosed as motor neuropathy, a disease process that can be acquired, hereditary […]
Man’s Face Paralyzed After Receiving Pfizer’s COVID Shot
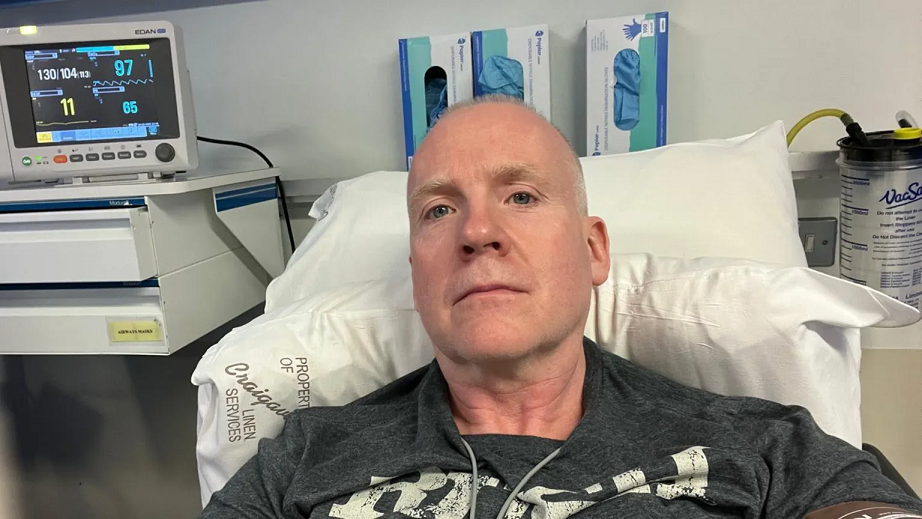
Larry Lowe, a 54-year-old resident of Omagh, Northern Ireland, experienced facial paralysis after receiving the Pfizer/BioNTech’s Comirnaty mRNA COVID-19 shot on Dec. 15, 2021. Prior to getting vaccinated, Lowe was healthy and physically active. Shortly after he receiving the shot, he developed numbness on the right side of his face and persistent pain. “I had […]
Triathlete Who Suffered Torn Coronary Artery Days After COVID Shots Undergoes More Surgery
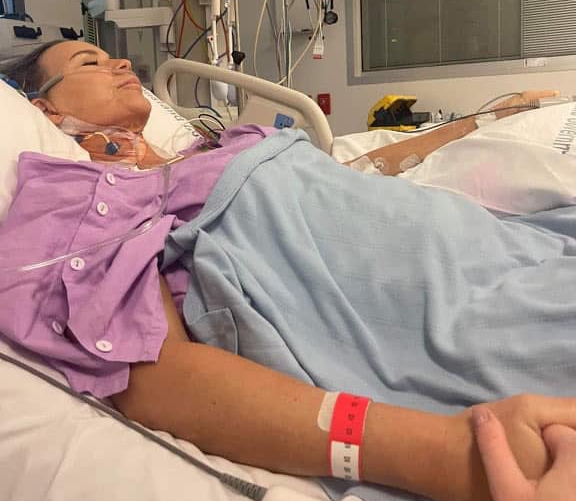
In mid-2021, triathlete and fitness instructor Ingi Doyle of Queensland, Australia received two doses of the Pfizer/BioNTech Comirnaty mRNA (messenger ribonucleic acid) COVID-19 biologic. The first shot on June 12, 2021 left her with a sore arm. After the second shot on July 4, she developed a severe adverse reaction that resulted in an aortic […]
Pfizer/BioNTech mRNA Combo Influenza and COVID Shot Efficacy Fails in Trial
Pfizer and BioNTech recently announced that a new mRNA vaccine designed to protect against both COVID-19 and influenza in a single shot faced a major setback after failing to achieve one of its primary goals in a late-stage trial. The “primary goal” missed in the late-stage trials was the ability to effectively prevent influenza B. […]
