Poll: 69 Percent of Americans Worried Fast-Tracked COVID-19 Vaccines Won’t Be Safe

Numerous polls have been conducted during the last few months asking Americans if they would get a COVID-19 vaccine were it approved by the U.S. government and offered to the public. Polls in April-May 2020 showed that about half or more of those surveyed said they would get the vaccine. More recent polling, however, has […]
Pfizer/BioNTech’s COVID-19 Vaccine Causes Adverse Reactions in Over Half of Clinical Trial Volunteers

In clinical trials of the experimental messenger RNA (ribonucleic acid) COVID-19 vaccine (BNT162b1), which is being developed by Pfizer, Inc. of New York and BioNTech SE of Mainz, Germany, adverse reactions have occurred in more than 50 percent of the adult participants. The Phase 1/2 human trials of BNT162b1 vaccine, which were conducted May-June 2020, […]
Infant Boy in India Dies Hours After Getting Oral Polio Vaccine

A seven-month-old infant boy died on July 7, 2020 within hours of receiving the live attenuated oral polio vaccine (OPV) in Palghar, India. The baby was given one dose of the OPV drops in the evening and was found dead the following morning by his parents.1 2 3 According to a news report, government welfare […]
81 Percent of Clinical Trial Volunteers Suffer Reactions to CanSino Biologics’ COVID-19 Vaccine That Uses HEK293 Human Fetal Cell Lines

An experimental vaccine for COVID-19 is being developed by CanSino Biologics, Inc. of Tianjin, China, in partnership with China’s Academy of Military Medical Sciences’ Institute of Biotechnology. A Phase 1 human clinical trial of the COVID-19 vaccine (adenovirus type-5 Ad5-nCoV) has been completed in China involving 108 healthy adults, ranging in age from 45–60 years […]
Pfizer and BioNTech Begin Human Clinical Trials of COVID-19 Vaccine in U.S.
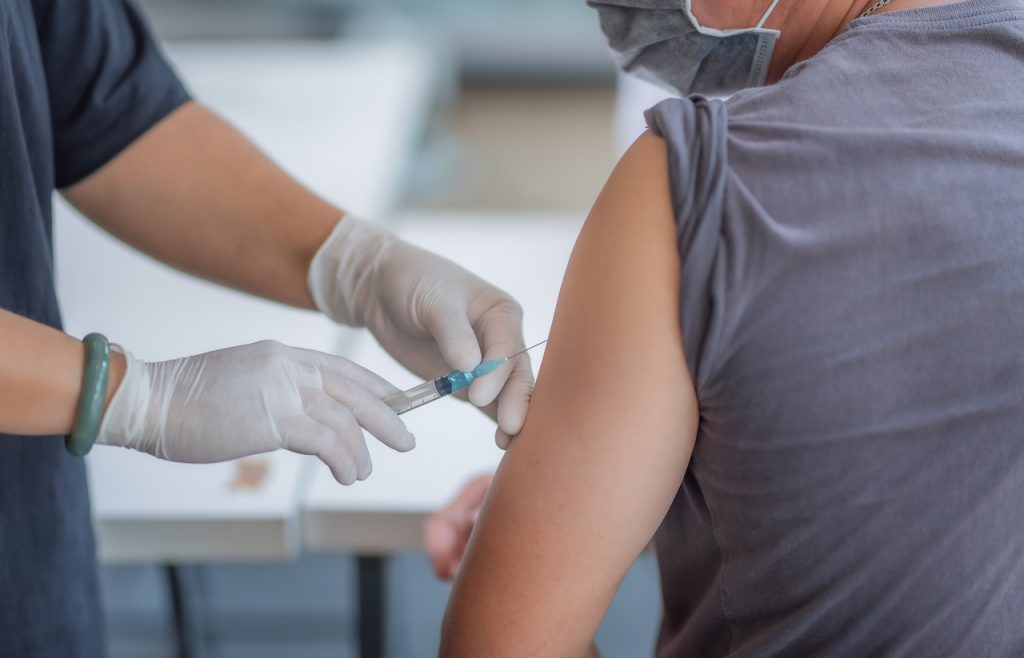
Pfizer, Inc. of New York and BioNTech SE of Mainz, Germany have announced that human clinical trials for an experimental COVID-19 vaccine they have co-developed, known as BNT162, have begun in the United States.1 As part of a global clinical development program, the first human subjects in the U.S. Phase I and Phase clinical II […]
Healthy Clinical Trial Subjects Suffer Grade 3 Side Effects to Moderna’s mRNA COVID-19 Vaccine

On May 18, 2020, Moderna, Inc. of Cambridge, Massachusetts announced that it had obtained “positive interim clinical data” from a Phase 1 human clinical trial of its experimental mRNA-1273 COVID-19 vaccine that began on Mar. 16. The biotechnology company said that eight of the 45 healthy adult volunteer trial subjects developed antibodies that may provide […]
