FDA’s Expert Panel Recommends COVID-19 Booster Dose for Seniors and Others, Rejects Booster Dose for Children

Despite not convening the Vaccines and Related Biologic Products Advisory Committee (VRBPAC) last month to vote on effectiveness and safety of the Pfizer-BioNTech COVID-19 vaccine (licensed under the name COMIRNATY), the U.S. Food and Drug Administration (FDA) convened the advisory committee on Friday, Sept. 17, 2021 to vote on booster doses of the vaccine.1 The […]
FDA Approves BioNTech’s Comirnaty. Pfizer COVID Shot Remains Experimental.
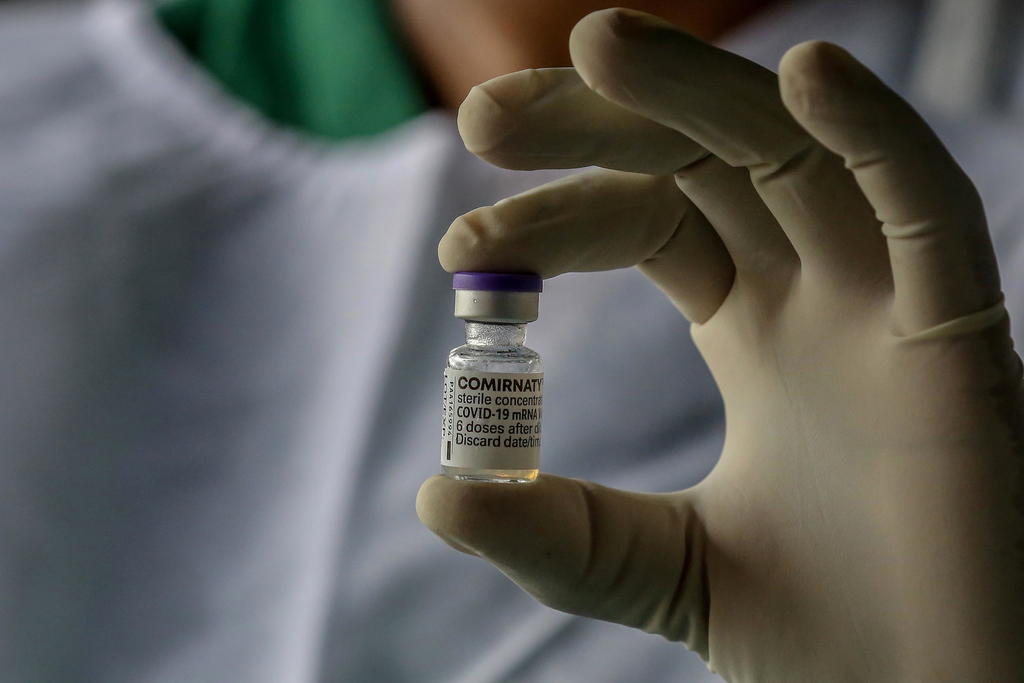
On Aug. 23, 2021, the U.S. Food and Drug Administration (FDA) gave full approval to a Biologics License Application (BLA) submitted by BioNTech Manufacturing GmbH of Mainz, Germany on May 18, 2021 for a biologic drug called COVID Vaccine, mRNA. The FDA gave permission to BioNTech to label the product “Comirnaty” and market it in […]
OB/GYN Docs in U.S. Want COVID-19 Vaccines Tested on Pregnant Women

A U.S. Centers for Disease Control and Prevention (CDC) report1 on pregnancy and SARS-CoV-2 infection released on June 26 suggested that pregnant women may be at increased risk for COVID-19 complications. The report was followed by a statement issued by the American College of Obstetricians and Gynecologists (ACOG)2 urging that pregnant and lactating women be […]
CDC Funds New Hampshire Vaccine Registry

New Hampshire’s Executive Council voted on Dec. 18, 2019 to accept $1.5 million from the Centers for Disease Control and Prevention (CDC) to create an Immunization Information System (IIS). According to the New Hampshire Department of Health and Human Services, New Hampshire is currently the only state without vaccine tracking system.1 The IIS electronic tracking […]

