‘COVID-19 Rebound’ May Be Worse Than Initial COVID Illness
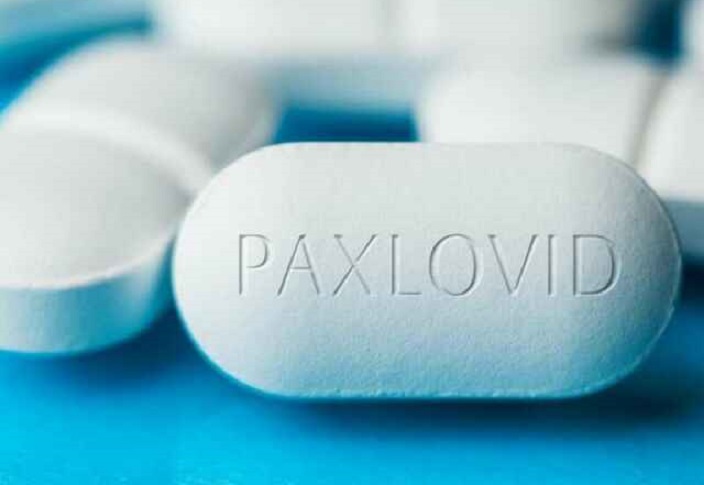
On May 24, 2022, the U.S. Centers for Disease Control and Prevention (CDC) issued a health advisory to health care providers, public health departments and the general public regarding Pfizer’s antiviral drug Paxlovid, designed to treat symptoms of COVID-19. The advisory warns that some people who take Paxlovid may experience a recurrence of COVID symptoms […]
Merck and Pfizer Seek Approval for COVID-19 Antiviral Drugs

Merck and Pfizer have submitted Emergency Use Authorization (EUA) applications to the U.S. Food and Drug Administration (FDA) to authorize distribution of what would be the first antiviral drug specifically designed to treat COVID-19 disease.1 Merck/Ridgeback Biotherapeutics are seeking EUA approval for Molnupiravir (MK-4482/EIDD-2801), an investigational oral antiviral drug originally developed to treat influenza.2 Pfizer […]
