Severe Reactions Minutes After COVID Vaccination Usually Written Off as Coincidental

The U.S. Centers for Disease Control and Prevention (CDC) recommends that everyone who receives a COVID-19 vaccine should be “monitored on site for at least 15 minutes after vaccination.” This is based on the belief that most allergic reactions to vaccines, including severe ones like anaphylaxis, which can lead to unconsciousness, seizures, shock, coma and […]
Another Study Confirms COVID Vaccine Alters Menstrual Cycles

A study published in the BMJ Medicine in August 2022 involving almost 20,000 women found an association between those who received a COVID-19 shot and an increased length in menstrual cycles. Of the 15,000 vaccinated women in the study, two-thirds received Pfizer/BioNTech’s COVID messenger RNA (mRNA) biologic, but the data also included women who received […]
Organ Transplant Failures After COVID Vaccination
In 2022, researchers have published articles in the medical literature reporting organ transplant failures in people who have recently received COVID-19 shots. These reports include corneal graft rejections and solid organ transplant failures involving the kidneys, livers, lungs, hearts and pancreas. A study by Japanese researchers published in June in the Journal of Clinical Medicine […]
Getting a Flu Shot and COVID Bivalent Booster Together

Despite President Biden’s recent admission that the COVID-19 pandemic is over, the White House is making another big push to encourage Americans to get yet another COVID booster shot. This time, the Biden administration is marketing the COVID Bivalent versions of messenger RNA (mRNA) Comirnaty and Spikevax biologics developed by Pfizer/BioNTech and Moderna/NIAID respectively.1 2 […]
Study Reveals Much Higher Risk for Serious Adverse Events With mRNA COVID Shots in Clinical Trials
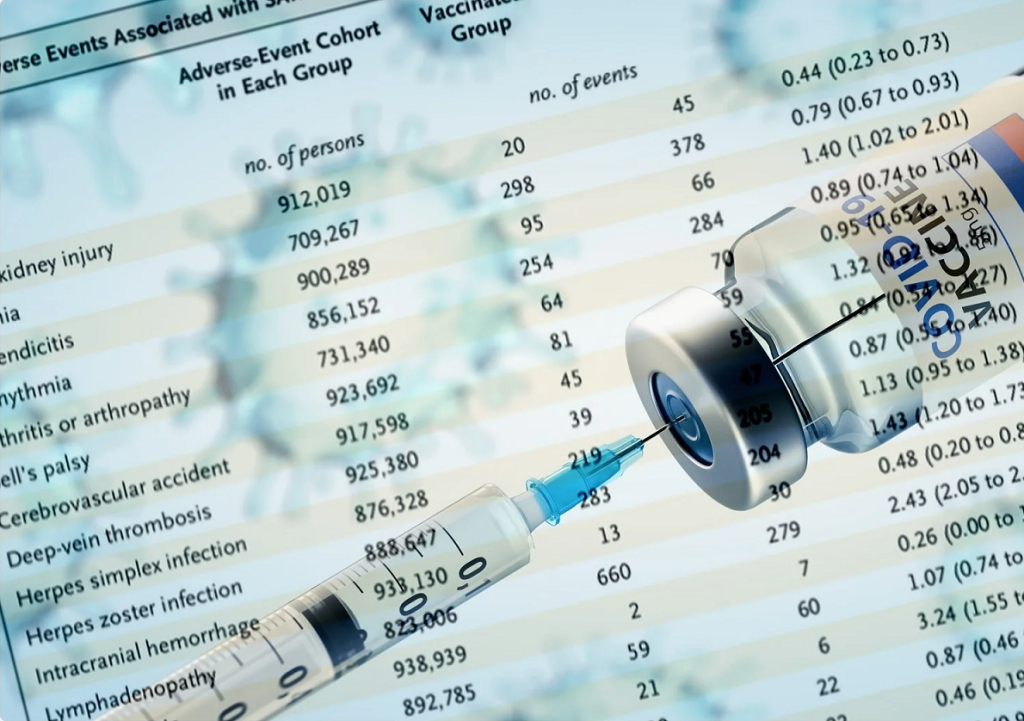
A new study published in the journal Vaccine revealed there was a much higher risk of serious adverse events of special interest for randomized clinical trial participants who received a messenger RNA (mRNA) COVID-19 biologic when compared to participants, who received a placebo for both the Pfizer/BioNTech mRNA BNT162b2 (also known as “Comirnaty”) COVID shot […]
Safety and Effectiveness of New COVID Bivalent Boosters Based on a Study of Eight Mice?

With no safety and efficacy evidence from human clinical trials, on Aug. 31, 2022, the U.S. Food and Drug Administration (FDA) amended the Emergency Use Authorizations (EUAs) for Pfizer/BioNTech’s Comirnaty and Moderna/NIAID’s Spikevax messenger RNA (mRNA) COVID-19 biologics to distribute “bivalent formulations” (two-in-one) of the shots to use as a “single booster dose.”1 2 The […]
