Developers of mRNA COVID Shots Spar Over Intellectual Property Rights
Pfizer and BioNTech will have to answer in court to legal claims by Moderna that they copied its innovative messenger RNA (mRNA) technology in the making of their COVID-19 biologic, Comirnaty. The patent infringement lawsuit filed in U.S. District Court for the District of Massachusetts and the Regional Court of Dusseldorf in Germany alleges that […]
German Report Reveals 1 in 25 Insured Individuals Treated for COVID Shot Reactions
Techniker Krankenkasse, Germany’s largest health insurance company, reported that 437,593 of 11 million (1 in 25) insured individuals in 2021 had to undergo medical treatment as a result of adverse reactions from COVID-19 shots.1 COVID shots currently approved for use in Germany include Novavax’s Nuvaxovid, Moderna’s Spikevax, Pfizer/BioNTech’s Comirnaty, Johnson & Johnson/Janssen’s Ad26.COV2.S, AstraZeneca/Oxford University’s […]
Pfizer CEO Tests Positive for COVID, Tweets He’s Grateful for Quadruple Vax and Paxlovid Treatment

Albert Bourla is the CEO of Pfizer and just announced on Twitter that he has COVID. He said, “I would like to let you know that I tested positive for COVID-19. I am thankful to have received four doses of the Pfizer-BioNTech vaccine, and I am feeling well while experiencing mild symptoms. I am isolating […]
Most American Parents Rejecting COVID Vaccine for Young Children

Despite the recommendation by the U.S. Centers for Disease Control and Prevention (CDC) that young children between the ages of six months and four years get vaccinated for COVID-19, only four to five percent of children in this age group have received the controversial mRNA COVID vaccines distributed by Pfizer/BioNTech and Moderna for administration to […]
Herpes Zoster Reactivated Following mRNA COVID Shots, Studies Show
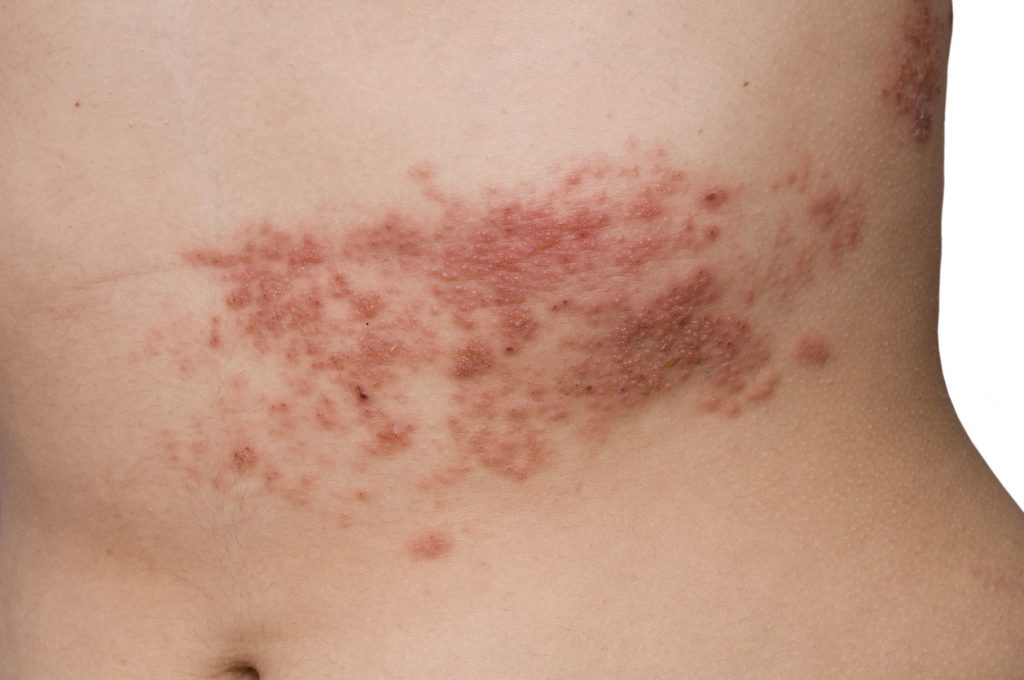
Concerns about the adverse effects of mRNA COVID-19 shots continue to grow a year and a half after they began to be distributed under an Emergency Use Authorization (EUA) granted to Pfizer/BioNTech and Moderna by the U.S. Food and Drug Administration (FDA). Cases of herpes zoster (HZ) following messenger RNA (mRNA) COVID shots have been […]
Nuvaxovid Offered as Alternative to mRNA Shots

For a virus (SARS-CoV-2) that many scientists and public health officials have maintained originated in a bat and accidently crossed over into humans, leading to the COVID-19 pandemic, it is odd that there does not appear to be much concern about producing vaccines that use genetic material from animals. You would think that this would […]
