Children Recruited in U.K. for COVID-19 Vaccine Trial

The University of Oxford’s Jenner Institute and Oxford Vaccine Group in the United Kingdom has announced that its researchers have begun recruiting children aged five to 12 years for phase II and phase III clinical trials testing an experimental COVID-19 vaccine the university is developing in partnership with AstraZeneca plc.1 Oxford’s ChAdOx1 nCov-19 (“chimpanzee adenovirus […]
COVID-19 Vaccine Will Likely Be Given Multiple Times, Perhaps Annually

In an interview with the JAMA on June 2, 2020, Anthony Fauci, MD, director of the U.S. National Institute of Allergy and Infectious Diseases (NIAID) said that while he was “cautiously optimistic” about the effectiveness of the vaccines being developed for COVID-19, he was concerned that any protection the vaccines may provide might only be temporary. […]
Pfizer and BioNTech Begin Human Clinical Trials of COVID-19 Vaccine in U.S.
Pfizer, Inc. of New York and BioNTech SE of Mainz, Germany have announced that human clinical trials for an experimental COVID-19 vaccine they have co-developed, known as BNT162, have begun in the United States.1 As part of a global clinical development program, the first human subjects in the U.S. Phase I and Phase clinical II […]
Healthy Clinical Trial Subjects Suffer Grade 3 Side Effects to Moderna’s mRNA COVID-19 Vaccine

On May 18, 2020, Moderna, Inc. of Cambridge, Massachusetts announced that it had obtained “positive interim clinical data” from a Phase 1 human clinical trial of its experimental mRNA-1273 COVID-19 vaccine that began on Mar. 16. The biotechnology company said that eight of the 45 healthy adult volunteer trial subjects developed antibodies that may provide […]
Live Tuberculosis “Vaccine” Being Tested as a Treatment for COVID-19 Infections
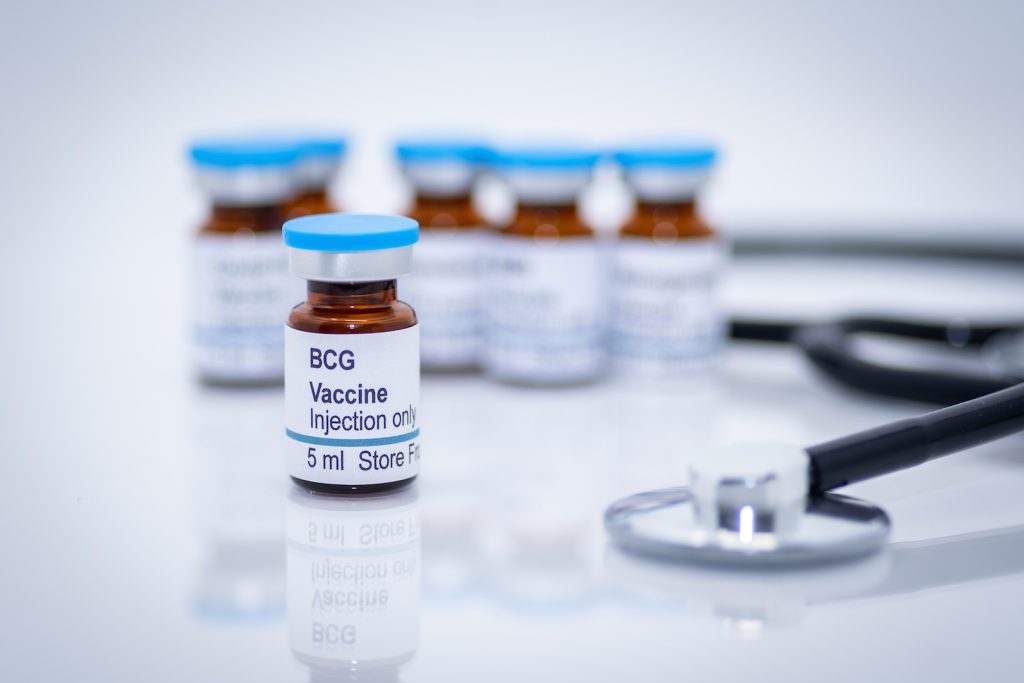
Story Highlights Researchers at Texas A&M University and five other academic institutions have begun a phase 4 clinical trial re-purposing a live attenuated tuberculosis vaccine to treat COVID-19. The researchers believe that the TICE® BCG vaccine, which is licensed to prevent and treat bladder cancer, may help mitigate the severity of COVID-19. The research team […]
Fauci Says Rushing COVID-19 Vaccine is Risky

During a remote online testimony before the U.S. Senate’s Health, Education, Labor and Pensions Committee on May 12, 2020, Anthony Fauci, MD warned that moving forward too quickly with the development of a vaccine for COVID-19 may produce a vaccine that could make the disease worse.1 2 Dr. Fauci, who is the director of the […]
