FDA Clears Investigational New Drug Application for Inhaled COVID Biologic
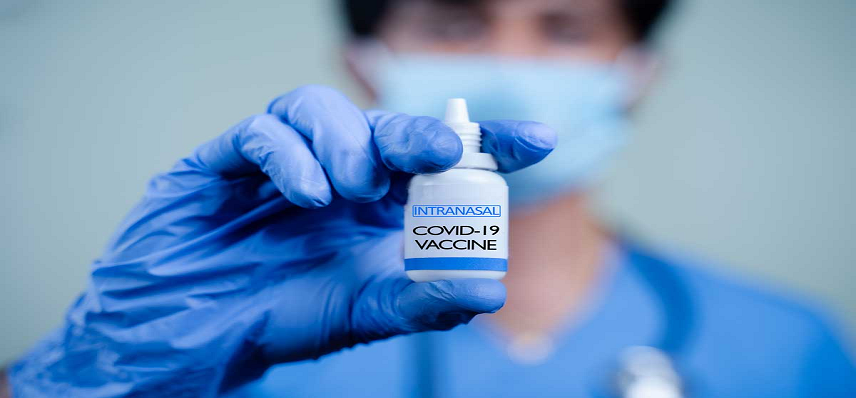
Biotech company Ocugen, Inc. has announced that the U.S. Food and Drug Administration (FDA) has cleared their Investigational New Drug (IND) application for a novel inhaled vaccine against SARS-CoV-2. The biologic uses novel chimpanzee adenovirus-vectored (ChAd36) technology. The approval will allow the company to initiate Phase I clinical trials for the inhaled vaccine, OCU500. The […]
COVID-19 Shot Safety, Efficacy Concerns Exposed by Florida Grand Jury
While recent news headlines proclaim that a Florida Grand Jury has found no evidence of crimes committed by the manufacturers of COVID-19 shots, the actual report published earlier this month paints a more thorough picture. Two years after Florida’s Governor Ron DeSantis called for a federal investigation into the experimental mRNA COVID biologics for “any […]
FDA Requires RSV Vaccine Labels to Include Guillain-Barré Syndrome Risk

On Jan. 7, 2025, the U.S. Food and Drug Administration (FDA) mandated and approved changes to the safety labeling for the Prescribing Information of two Respiratory Syncytial Virus (RSV) vaccines: Abrysvo (manufactured by Pfizer)1 and Arexvy (manufactured by GlaxoSmithKline Biologicals).2 This label change update is being required to inform both the public and health care […]
Moderna Halts RSV Pediatric Vaccine Trial After Five Infants Hospitalized
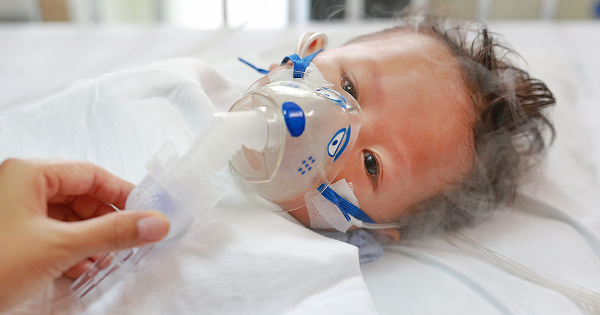
On Dec. 11, 2024, the U.S. Food and Drug Administration (FDA) announced that clinical trials for Moderna’s pediatric mRNA RSV vaccines have been paused due to safety concerns. The recent phase 1 trials of two experimental respiratory syncytial virus (RSV) vaccines for infants not only failed to provide adequate protection but may have worsened respiratory […]
Louisiana Bans State Funded Promotion of COVID-19, Influenza and Mpox Vaccines

In December 2024, Louisiana’s Department of Health issued a directive barring public health workers from promoting COVID-19, influenza, or mpox vaccines.1 This includes prohibiting staff from sending press releases, giving interviews, holding vaccine events, creating social media posts, or even displaying signs in clinics indicating that vaccines are available.2 The policy, which officials declined to […]
Topical Cream Vaccine Created by Stanford University Scientists
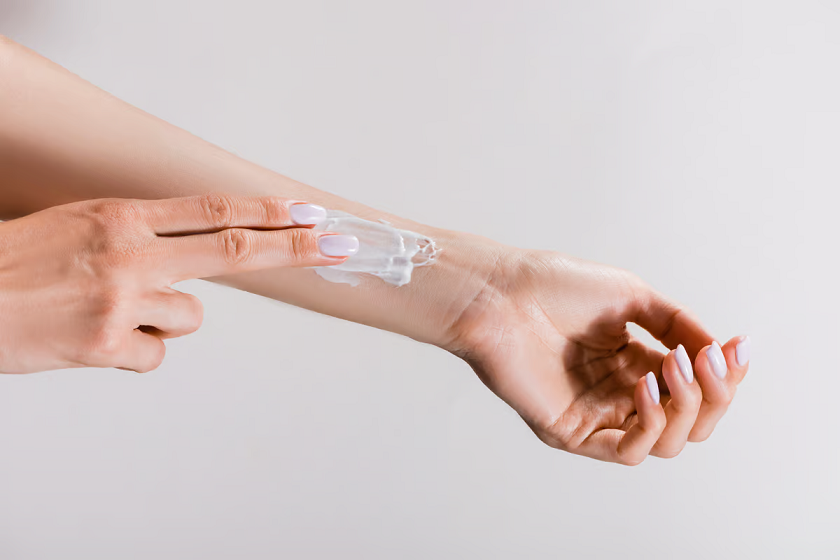
A new study conducted by Stanford University scientists and published in Nature discusses a newly developed topical vaccine using Staphylococcus epidermidis, a common bacterium found on human skin. The researchers speculate that this new type of vaccine delivery system could offer an alternative to traditional needle-based vaccinations and avoid common side effects like fever, swelling, […]
