Novel mRNA Lyme Shots Could Be Released in 2025

Two novel messenger RNA (mRNA) shots for Lyme disease currently in late-stage clinical trials could hit the market as early as 2025, Moderna announced earlier this year. Also in the race to develop the first-ever mRNA product to prevent Lyme disease are Pfizer and Austria-based Valneva, which entered into a collaborative agreement in April 2020 […]
3D Printed Vaccine Skin Patches Being Developed
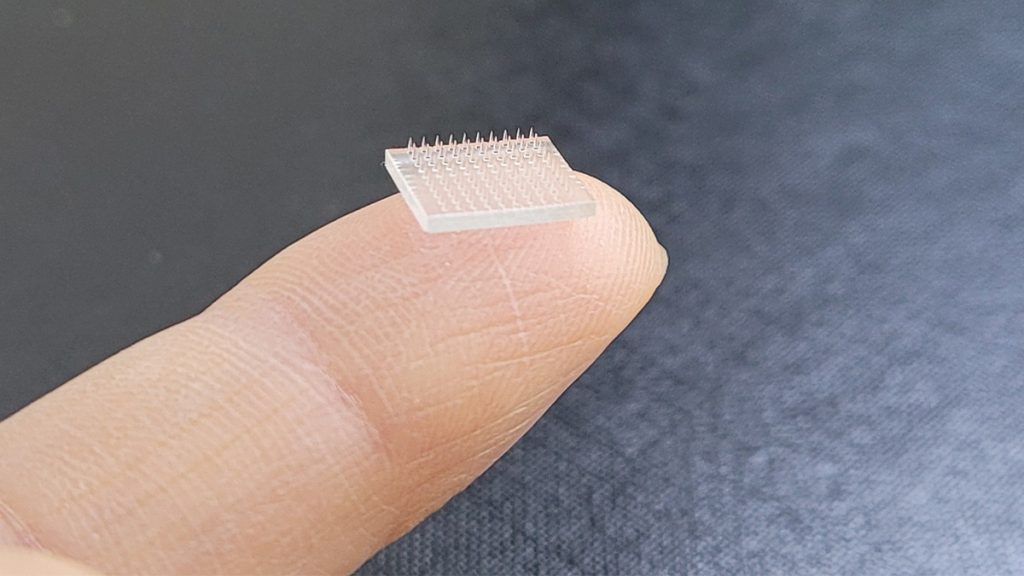
In an attempt to increase consumer demand for COVID-19 shots, researchers have designed a vaccine printer that can print COVID messenger RNA (mRNA) skin patches in bulk, producing as many as 100 patches in 48 hours. The microneedle patches, which contain mRNA molecules in lipid nanoparticles, could pave the way for unlimited distribution of the […]
U.S. to Spend Billions on New COVID Vaccines Despite Failure to Prevent Infection
The Biden administration announced earlier this month that the U.S. government will spend at least $5 billion on Project NextGen to rapidly develop “next-generation” SARS-CoV-2 vaccines and treatments despite evidence that current COVID vaccines do not prevent infection and transmission of the new coronavirus.1 Following a similar approach to “Operation Warp Speed” initiated by the […]
Pharma Developing Bird Flu Vaccine for Humans As Animals Get Sick
Pharmaceutical companies GSK (formerly known as GlaxoSmithKline), Moderna and CSL Seqirus announced that they are developing and ready to test human vaccines against avian influenza H5N1 (also known as bird flu) as a precautionary measure to prepare for what they believe may be a future pandemic.1 Now that certain strains of H5N1 have infected not […]
Group B Strep Vaccine for Pregnant Women on Fast Track for FDA Approval

Pfizer is conducting phase 2 trials for the development of a vaccine that will target pregnant women to prevent newborns from infection with Group B Streptococcal (GBS). The vaccine was granted Breakthrough Therapy Designation by the U.S. Food and Drug Administration (FDA) in September and the agency has indicated it will fast track the product’s […]
Moderna, Novavax Developing Combined Single-Dose COVID-19 and Influenza Vaccine
American pharmaceutical and biotechnology company Moderna, Inc., which partnered with the U.S. National Institutes of Allergy and Infectious Diseases (NIAID) to develop an mRNA COVID-19 vaccine being distributed in the United States under an Emergency Use Authorization (EUA) announced that it is developing a combined single-dose booster vaccine for COVID-19 and seasonal influenza.1 In a […]
