Improving Science & Restoring Trust in Public Health | Jay Bhattacharya, MD

A substantial part of the NIH portfolio, appropriately, focuses on basic science. Basic science meaning fundamental biological facts that can be used in many many drug studies, other research where you don’t necessarily know specifically in advance when you’re doing it what the application’s going to be. The NIH, very appropriately, funds that work, especially […]
New Jersey Bill Requires Automatic Enrollment in State Electronic Vaccine Tracking Registry

Lawmakers in New Jersey have approved legislation that would require enrollment in the state’s electronic vaccine tracking registry, unless citizens actively opt out in writing. The bill for an “opt-out” vaccine tracking system, rather than operating an “opt-in” system, comes in response to decreased vaccination rates in recent years and health officials maintain it will […]
Clinical Trials Now Required for Annual COVID Shots Given to Healthy Adults

On May 20, 2025, the U.S. Food and Drug Administration (FDA) announced that new clinical trials will be required to approve annual COVID-19 booster shots for healthy adults under age 65. In a joint editorial published in the New England Journal of Medicine, FDA commissioner Marty Makary, MD and the director of the FDA’s Center […]
Know the Risks of Rhogam and Vitamin K Shots Given to Pregnant Women and Newborn Babies

Pregnant mothers and newborn babies are routinely given a number of shots. Two controversial shots are Rhogam and vitamin K. Since the 1970’s, mothers-to-be whose blood work shows that they are Rh factor (Rhesus factor) negative are told to get a shot of Rh immunoglobulin (RhoGAM) during pregnancy, as well as after delivery if the […]
CDC Vaccine Advisory Panel Purged

On June 9, 2025, U.S. Secretary of Health and Human Services Robert F. Kennedy, Jr. fired all 17 members of the U.S. Centers for Disease Control and Prevention’s (CDC) committee that makes national vaccine policy—the Advisory Committee on Immunization Practices (ACIP). “A clean sweep is necessary to reestablish public confidence in vaccine science,” Secretary Kennedy […]
FDA Approves MenQuadfi Meningococcal Vaccine for Six-Week-Old Infants
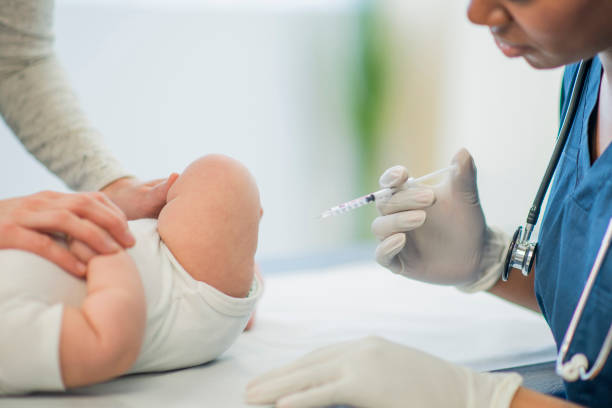
The U.S. Food and Drug Administration (FDA) has licensed Sanofi’ SA’s four-valent MenQuadfi meningococcal vaccine for use in infants aged six weeks to 23 months old to prevent invasive meningococcal disease. Previously approved for children aged two and older, MenQuadfi is now the first meningococcal vaccine included on the U.S. Centers for Disease Control and […]
