Moderna Withdraws Application for Combination COVID and Flu Vaccine

Moderna, Inc. withdrew its application for United States licensure for its combination COVID-19/Influenza vaccine known as mRNA-1083. The company stated the decision came “in consultation with” the U.S. Food and Drug Administration (FDA).1 The decision came just one day after the FDA announced the agency would require new clinical trials for approval of annual COVID […]
Flu Shot Recipients “More Likely” to Get Influenza Than Unvaccinated
The American Medical Association (AMA) cites that the influenza vaccine is estimated to be 40-60 percent effective at preventing serious infections or death from the influenza virus that affects up to 40 million Americans every year. However, for the 2024-2025 flu “season” the influenza vaccine not only missed the mark on effectiveness, but is linked […]
FDA Requires RSV Vaccine Labels to Include Guillain-Barré Syndrome Risk

On Jan. 7, 2025, the U.S. Food and Drug Administration (FDA) mandated and approved changes to the safety labeling for the Prescribing Information of two Respiratory Syncytial Virus (RSV) vaccines: Abrysvo (manufactured by Pfizer)1 and Arexvy (manufactured by GlaxoSmithKline Biologicals).2 This label change update is being required to inform both the public and health care […]
Moderna Halts RSV Pediatric Vaccine Trial After Five Infants Hospitalized
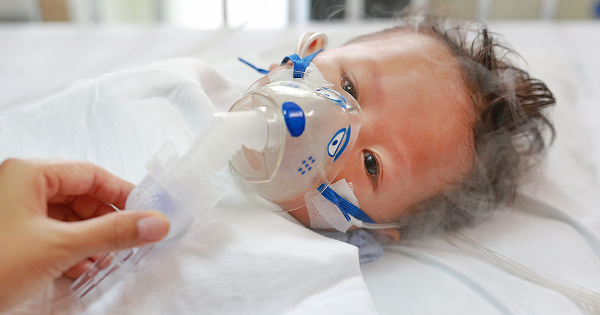
On Dec. 11, 2024, the U.S. Food and Drug Administration (FDA) announced that clinical trials for Moderna’s pediatric mRNA RSV vaccines have been paused due to safety concerns. The recent phase 1 trials of two experimental respiratory syncytial virus (RSV) vaccines for infants not only failed to provide adequate protection but may have worsened respiratory […]
WHO Names 17 Pathogens as Top Priorities for Global Vaccine Development

A new World Health Organization (WHO) study published in eBioMedicine last week highlights 17 viral and bacterial pathogens that are major sources of endemic disease around the world and that WHO officials believe should be urgently prioritized for vaccine development. The study marks the WHO’s first global effort to systematically prioritize endemic pathogens with epidemic […]
CDC Narrows RSV Vaccine Guidelines Due to Safety Concerns
The U.S. Centers for Disease Control and Prevention (CDC) has recently narrowed its original recommendations for the respiratory syncytial virus (RSV) vaccine, citing concerns over the risk of a side effect that can lead to paralysis or death. The recommendations originally stated adults over the age of 60 should receive the vaccine, but updates in […]
