Jacking Up the Price of Lemons: The COVID Vaccine Scam

In July 2020, the U.S. government ordered 100 million doses of Pfizer/BioNTech’s experimental BNT162b2 (now known as Comirnaty) messenger RNA (mRNA) COVID-19 biologic for $1.95 billion, or $19.50 per dose. A few months later in December, it followed up with a second order for another 100 million doses of the shot for the same price.1 […]
Thyroid Disorders Can Be Caused or Worsened by COVID Shots
There have been a number of reports published in the medical literature describing cases of thyroid autoimmunity developing in individuals, who have received different types of COVID-19 shots.1 Cases occur in individuals who have never been diagnosed with a thyroid disorder and in those whose thyroid disorders worsen after COVID vaccinations. The thyroid is an […]
Study Reveals Much Higher Risk for Serious Adverse Events With mRNA COVID Shots in Clinical Trials
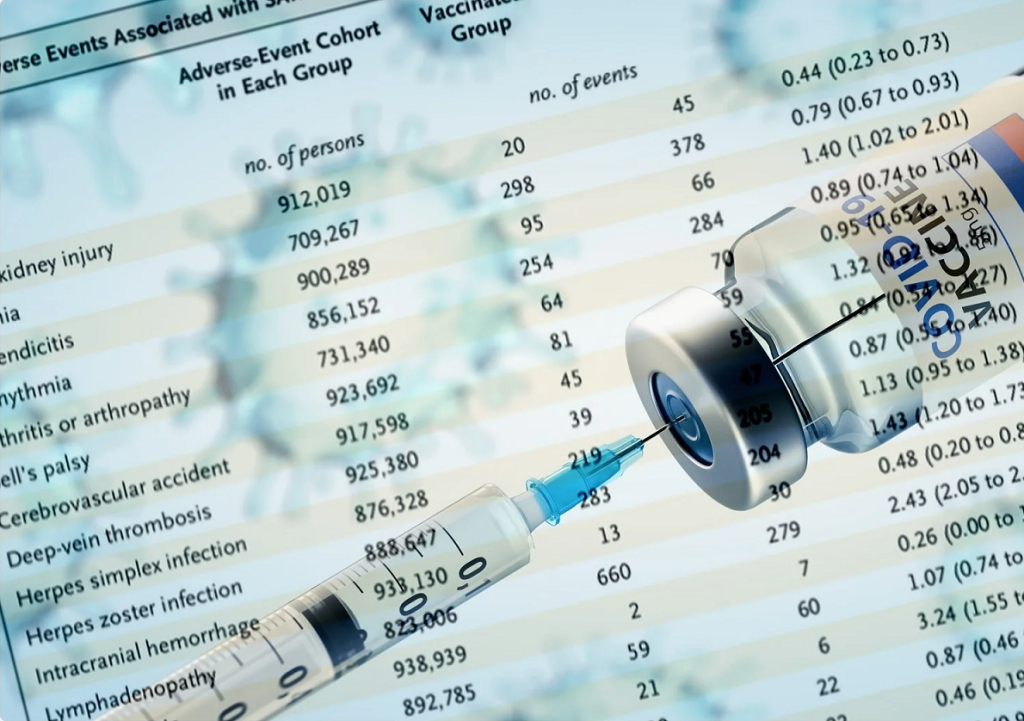
A new study published in the journal Vaccine revealed there was a much higher risk of serious adverse events of special interest for randomized clinical trial participants who received a messenger RNA (mRNA) COVID-19 biologic when compared to participants, who received a placebo for both the Pfizer/BioNTech mRNA BNT162b2 (also known as “Comirnaty”) COVID shot […]
Herpes Zoster Reactivated Following mRNA COVID Shots, Studies Show
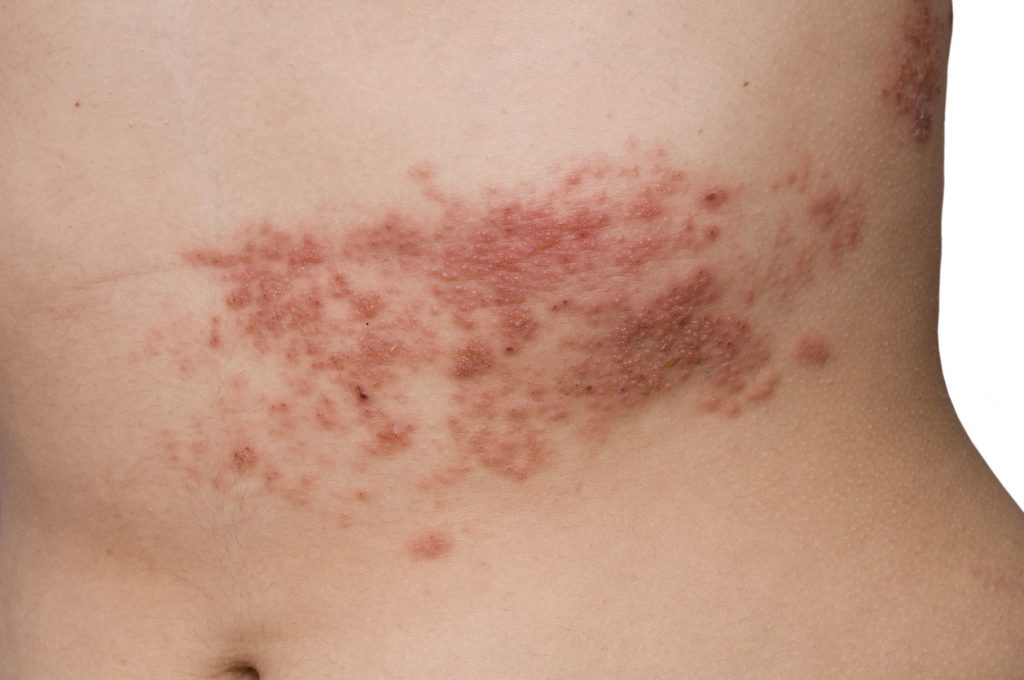
Concerns about the adverse effects of mRNA COVID-19 shots continue to grow a year and a half after they began to be distributed under an Emergency Use Authorization (EUA) granted to Pfizer/BioNTech and Moderna by the U.S. Food and Drug Administration (FDA). Cases of herpes zoster (HZ) following messenger RNA (mRNA) COVID shots have been […]
FDA Grants EUA for Novavax’s COVID Vaccine
Opinion | On July 13, 2022, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) to Novavax to distribute its experimental two-dose NVX-CoV2373 (also known as “Nuvaxovid” and “Covovax”) COVID-19 vaccine for use by adults. The decision was expected, given the favorable recommendation by the FDA’s Vaccines and Related Biological Products Advisory […]
Study Shows Sperm Concentration Decreased Three Months Following Pfizer’s COVID-19 Vaccinations
In a new study published in the journal Andrology, researchers found what they believe to be a temporary decline of sperm concentration and total motile count among sperm donors in Israel three months following vaccination with Pfizer/BioNTech’s BNT162b2 (also known as “Comirnaty”) messenger RNA (mRNA) COVID-19 biologic.1 Study Investigates the Effect of the Pfizer COVID […]
