Merck and Pfizer Seek Approval for COVID-19 Antiviral Drugs

Merck and Pfizer have submitted Emergency Use Authorization (EUA) applications to the U.S. Food and Drug Administration (FDA) to authorize distribution of what would be the first antiviral drug specifically designed to treat COVID-19 disease.1 Merck/Ridgeback Biotherapeutics are seeking EUA approval for Molnupiravir (MK-4482/EIDD-2801), an investigational oral antiviral drug originally developed to treat influenza.2 Pfizer […]
FDA, CDC Green Light Pfizer’s Experimental COVID Biologic for Children 5 to 11 Years Old
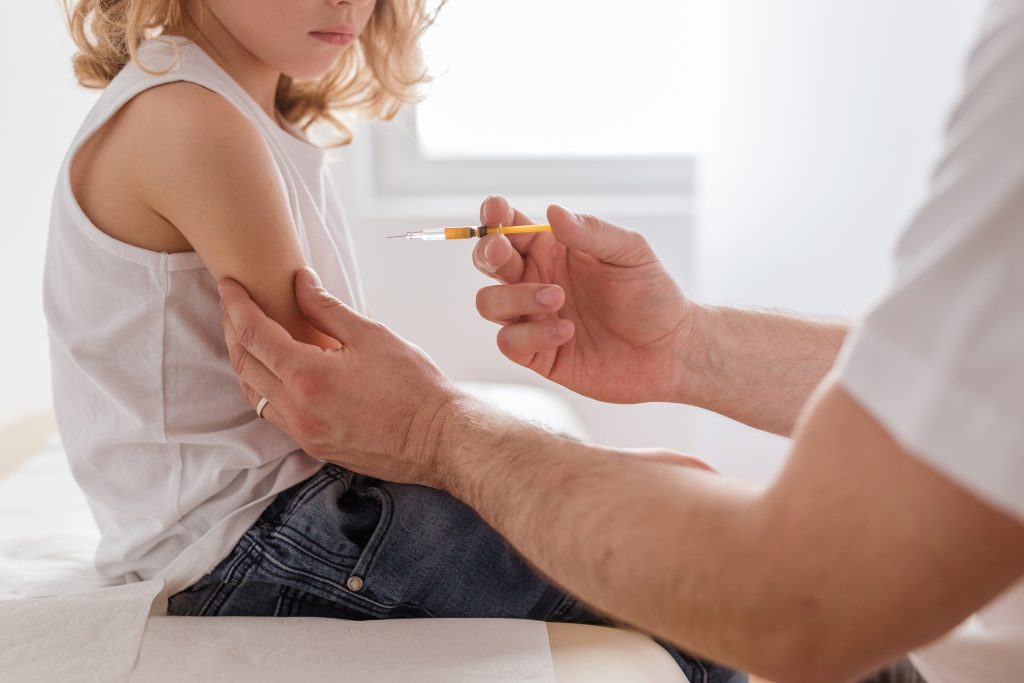
One Oct 29, 2021, the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for the experimental messenger RNA (mRNA) Pfizer/BioNTech COVID-19 biologic BNT162b2 for children five to 11 years old. The FDA’s decision, which is based on a clinical trial earlier this year involving 2,268 kindergartners and elementary school-aged kids and an […]
White House Reveals Plans to Give COVID Shots to All 5 to 11 Year Old Children

On Oct. 20, 2021, the Biden administration outlined plans to begin giving COVID-19 vaccinations to children between five and 11 years old. Pending the results of reviews of the plan by the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) over the […]
Study Finds COVID Cases Unrelated to Vaccination Rates

As vaccines and mandates continue to be the primary means of combating COVID-19, findings from a recently published article demonstrate that COVID-19 cases across 68 countries and nearly 3,000 counties in the United States were unrelated to vaccination rates, indicating “a lack of meaningful association.”1 The international data, published in the European Journal of Epidemiology, […]
Washington Mom Dies of Blood Clotting Syndrome 12 Days After J&J COVID Vaccination

A 37-year-old woman in King County, Washington died unexpectedly on Sept. 7, 2021, just 12 days after receiving Johnson & Johnson/Janssen’s experimental Ad26.COV2.S vaccine for COVID-19. Jessica Berg Wilson, the mother of two young daughters, was vaccinated on Aug. 26 and subsequently developed a blood clotting syndrome, known as “thrombosis with thrombocytopenia syndrome” (TTS), that […]
NIH Will Fund Studies on Possible Link Between COVID Vaccination and Menstrual Cycle Changes

According to the British Medical Journal (BMJ), more than 30,000 British women experienced changes to their menstrual cycle after getting the COVID-19 vaccine. Reproductive immunologist Victoria Male, PhD wrote an opinion piece for BMJ calling for more research on the subject and stating, “a link is plausible and should be investigated.”1 Reported changes to women’s […]
