Basketball Player Dies of Heart Attack After Two Pfizer COVID Shots
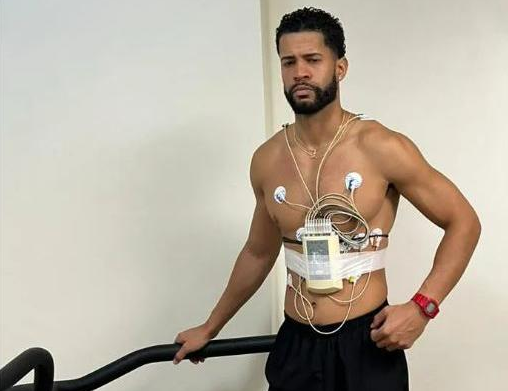
Óscar Cabrera Adames, a 28-year-old professional basketball player from the Dominican Republic, suffered a heart attack and died while performing a stress test at a health center. A stress test measures the electrical activity of the heart during physical activity. It has not yet been conclusively determined whether undergoing the stress test is what led […]
“Long COVID Vaccination” Syndrome and “Long COVID” Illness are Similar

Researchers have been working for several years on understanding why some individuals suffer a pattern of persistent health problems after being infected with SARS-CoV-21 2 or after COVID vaccination.3 4 5 Both long COVID syndrome and long post-COVID vaccination syndrome (LPVCS) are associated with similar debilitating symptoms that affect immune and brain function.6 Although U.S. […]
Meta Review of Autopsies Finds Link Between COVID Shots and Death
An independent meta review of autopsies on people who died after COVID-19 vaccination, published as a preprint in The Lancet online on July 5, 2023, found that 74 percent of the deaths were causally related to the shots. The review included 44 published papers that contained 325 autopsy cases and one necropsy case. According to […]
Some Children Suffered Seizures Following mRNA COVID Shots

A 2023 analysis published by researchers from Kaiser Permanente and the U.S. Centers for Disease Control and Prevention (CDC) in the journal Pediatrics compared vaccine adverse outcomes following administration of Pfizer/BioNTech’s Comirnaty messenger RNA (mRNA) COVID-19 shot and Moderna/NIAID’s Spikevax mRNA COVID-19 shot among recipients under five years old for a period of 1-21 days […]
BioNTech Sued in Germany for Injuries from the Comirnaty COVID mRNA Shot
Pharmaceutical company BioNTech SE of Mainz, Germany is facing a lawsuit filed by a German health care worker who was injured by the company’s Comirnaty COVID-19 messenger RNA (mRNA) shot, which it developed in collaboration with Pfizer, Inc. of the United States. The plaintiff said she suffered pain in her upper body, extreme swelling, fatigue […]
Some Doctors Say mRNA COVID Shots May Cause Tinnitus

Following the publication of a study in the journal JAMA Otolaryngology-Head & Neck Surgery on Feb. 24, 2022 that found a possible association between Pfizer/BioNTech’s Comirnaty messenger RNA (mRNA) COVID-19 shot and sudden sensorineural hearing loss (SSNHL), The Vaccine Reaction published an article about the study. The retrospective cohort study involved 2 ,602, 557 patients in Israel […]

