First Severe Case of Human Bird Flu Confirmed in U.S.

Officials at the U.S. Centers for Disease Control and Prevention (CDC) have confirmed diagnosis of the first severe human case of “bird flu” or H5N1 avian influenza. They determined that the hospitalized patient, a resident of Louisiana, was exposed to “sick and dead birds in backyard flocks,” although the source of the infection in the […]
U.S. Government Gives Moderna $176 Million to Develop mRNA Bird Flu Biologic
On July 2, 2024, the U.S. government awarded a $176 million contract to Moderna, Inc. for the development of an mRNA H5N1 avian influenza biologic labeled a “vaccine,” as the media reports concerns about bird flu in dairy cows and farm workers in multiple states. The funds from the U.S. Biomedical Advanced Research and Development […]
BARDA Awards $9.27 Million Contract to Evaluate Oral Pill COVID Vaccine

Biotechnology firm Vaxart, Inc. announced on Jan. 19, 2024 that it received a $9.27 million contract from the U.S. Biomedical Advanced Research and Development Authority (BARDA) to fund preparations for a Phase IIb clinical trial to evaluate the company’s oral pill XXB COVID-19 vaccine candidate. The trial is expected to involve 10,000 participants and will […]
3D Printed Vaccine Skin Patches Being Developed
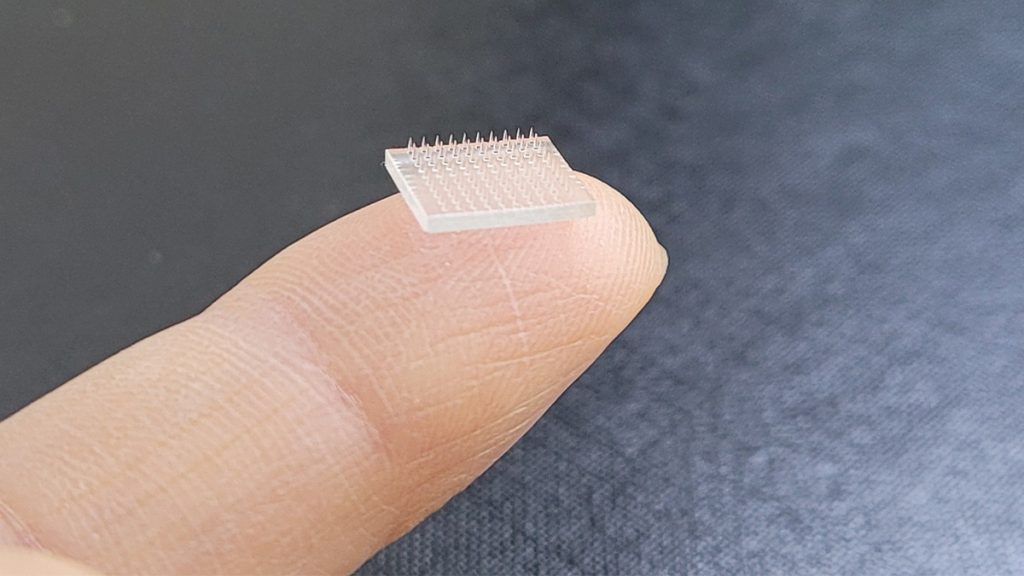
In an attempt to increase consumer demand for COVID-19 shots, researchers have designed a vaccine printer that can print COVID messenger RNA (mRNA) skin patches in bulk, producing as many as 100 patches in 48 hours. The microneedle patches, which contain mRNA molecules in lipid nanoparticles, could pave the way for unlimited distribution of the […]
Smallpox Vaccine Manufacturers See Big Stock Price Increases After Monkeypox Outbreaks Reported
On May 26, 2022, U.S. Centers for Disease Control (CDC) officials held a press briefing and announced there had been nine cases of monkeypox identified in seven U.S. states and that two licensed smallpox vaccines were in the national stockpile and ready to be distributed.1 The CDC’s director said, “The U.S. has the resources we […]
Monkeypox Cases in U.S. Coincide With Vaccine Purchases by BARDA
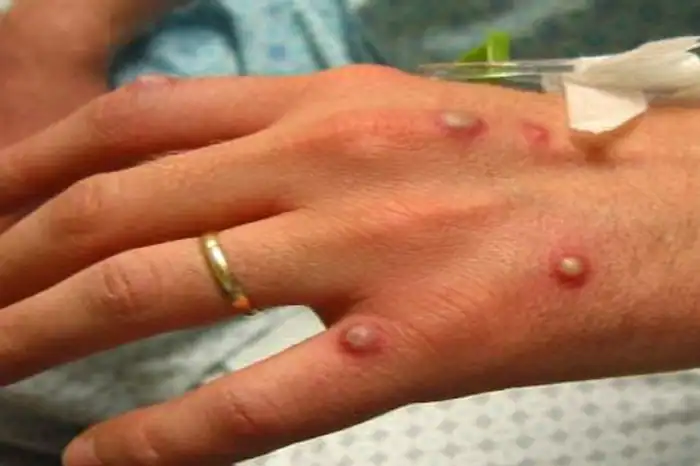
The World Health Organization (WHO) announced on May 21, 2022 that more than 100 confirmed or suspected cases of monkeypox had been identified in 12 countries, including between one and five confirmed cases in the U.S.1 Most of the cases have occurred in Europe with higher numbers in Portugal, Spain and the U.K. WHO officials […]
