Clinical Trials Now Required for Annual COVID Shots Given to Healthy Adults

On May 20, 2025, the U.S. Food and Drug Administration (FDA) announced that new clinical trials will be required to approve annual COVID-19 booster shots for healthy adults under age 65. In a joint editorial published in the New England Journal of Medicine, FDA commissioner Marty Makary, MD and the director of the FDA’s Center […]
CDC Vaccine Advisory Panel Purged

On June 9, 2025, U.S. Secretary of Health and Human Services Robert F. Kennedy, Jr. fired all 17 members of the U.S. Centers for Disease Control and Prevention’s (CDC) committee that makes national vaccine policy—the Advisory Committee on Immunization Practices (ACIP). “A clean sweep is necessary to reestablish public confidence in vaccine science,” Secretary Kennedy […]
FDA Approves MenQuadfi Meningococcal Vaccine for Six-Week-Old Infants
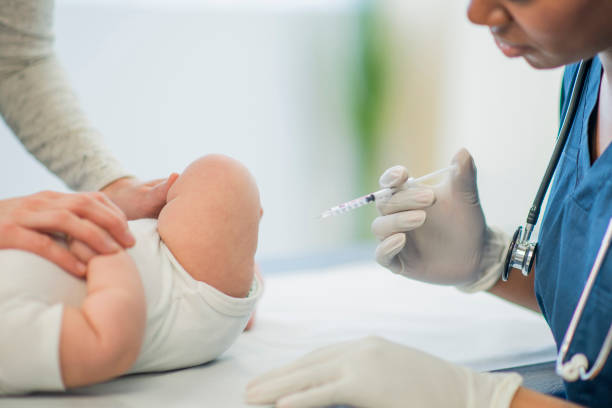
The U.S. Food and Drug Administration (FDA) has licensed Sanofi’ SA’s four-valent MenQuadfi meningococcal vaccine for use in infants aged six weeks to 23 months old to prevent invasive meningococcal disease. Previously approved for children aged two and older, MenQuadfi is now the first meningococcal vaccine included on the U.S. Centers for Disease Control and […]
Government Cancels $700 Million in Funding for Moderna’s Bird Flu Biologic

The U.S. government has cancelled a contract worth over $700 million awarded to Moderna, Inc. for late-stage development of an mRNA bird flu biologic for humans, as well as the right to purchase the shots. Earlier this year, the Department of Health and Human Services (DHHS) Biomedical Advanced Research and Development Authority (BARDA) issued the […]
FDA Licenses Moderna’s mNEXSPIKE COVID Shot for Individuals at High Risk for Severe COVID Disease
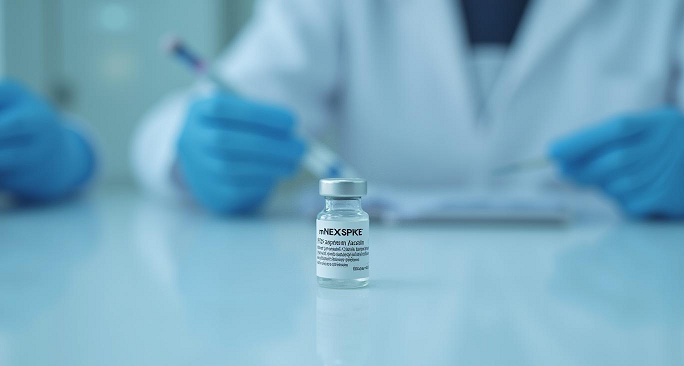
On May 30, 2025, the U.S. Food and Drug Administration (FDA) licensed mNEXSPIKE (mRNA-1283), a newly developed mRNA COVID-19 biologic for individuals who have previously received any COVID shots and are either 65 years of age or older, or between 12 and 64 years old with at least one underlying medical condition that increases their […]
Moderna Withdraws Application for Combination COVID and Flu Vaccine

Moderna, Inc. withdrew its application for United States licensure for its combination COVID-19/Influenza vaccine known as mRNA-1083. The company stated the decision came “in consultation with” the U.S. Food and Drug Administration (FDA).1 The decision came just one day after the FDA announced the agency would require new clinical trials for approval of annual COVID […]
