Doctor Sued for Coercing Minor Children to Get Vaccinated
A mother in Washington, DC is suing a doctor for allegedly forcing her teenaged daughter and son to receive COVID-19 shots without her informed consent. NaToya McNeil claims that Janine Rethy, MD, MPH, chief of community pediatrics at MedStar Georgetown University Hospital, isolated her children in a room in a mobile clinic and refused to […]
Taiwan Compensates Family of Girl Who Died Days After Getting Second Dose of Pfizer’s COVID Shot
The Ministry of Health and Welfare of Taiwan has agreed to pay $114,829 under the National Vaccine Injury Compensation Program (VICP) to the family of a young girl in Taoyuan, Taiwan, who died of myocarditis (inflammation of the heart muscle) within days of receiving the second dose of Pfizer/BioNTech’s Comirnaty COVID-19 messenger RNA (mRNA) shot. […]
COVID-19 Vaccine Injury Compensation Program Described as Legal Black Hole

Attorneys familiar with vaccine injury claims have described the current compensation program for COVID-19 vaccines as a legal “black hole” and inadequate for addressing the vaccine injury claims associated with the fast-tracked COVID vaccines.1 Attorney Altom Maglio says he has been contacted by hundreds of people who suspect they have suffered COVID vaccine-related injuries but […]
Claims for Injury, Death from COVID Shots Surge

Opinion | The Countermeasures Injury Compensation Program (CICP) is a program of the U.S. government that provide financial compensation for people who have suffered serious injuries or death from vaccines, biologics, antivirals or other drugs, as well medical devices used to “diagnose, prevent or treat a declared pandemic, epidemic or security threat.” The program was […]
FDA Approves BioNTech’s Comirnaty. Pfizer COVID Shot Remains Experimental.
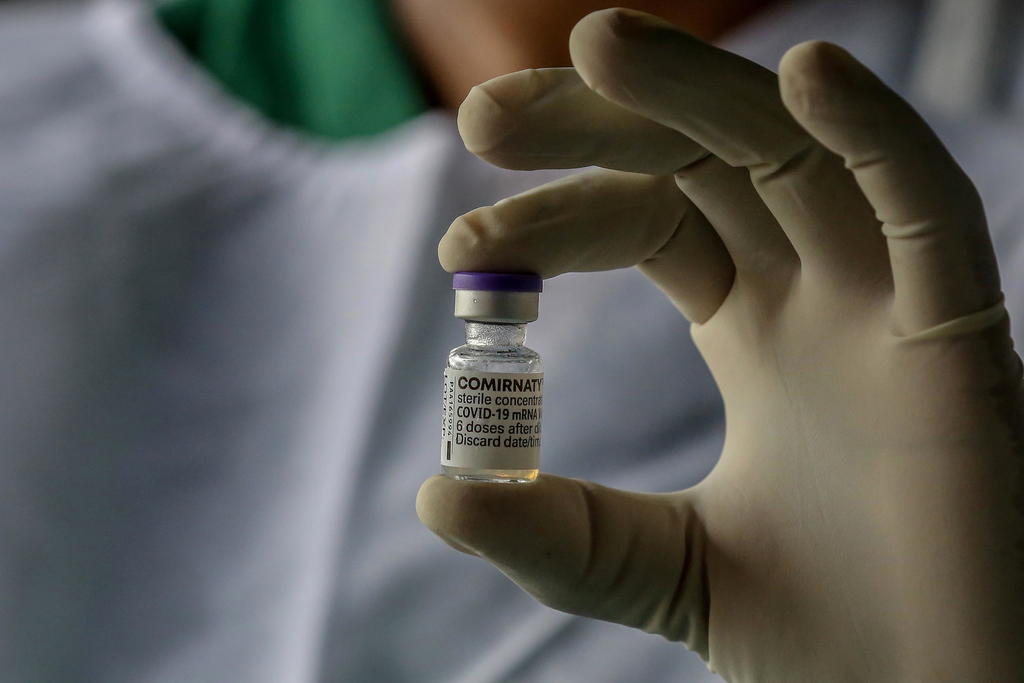
On Aug. 23, 2021, the U.S. Food and Drug Administration (FDA) gave full approval to a Biologics License Application (BLA) submitted by BioNTech Manufacturing GmbH of Mainz, Germany on May 18, 2021 for a biologic drug called COVID Vaccine, mRNA. The FDA gave permission to BioNTech to label the product “Comirnaty” and market it in […]
COVID-19 Vaccine Trial Volunteer in India Develops Encephalopathy and Sues

A 40-year old business consultant in India, who volunteered to be a participant in clinical trials of the experimental COVID-19 vaccine (Covishield) created by AstraZeneca and Oxford University is suing Serum Institute of India (SII), which is producing the vaccine in India, for 50 million rupees ($680,000) after he developed acute encephalopathy (brain dysfunction) within […]

