FDA, CDC Halt Use of Chikungunya Vaccine in Adults 60+ After Two Deaths Reported
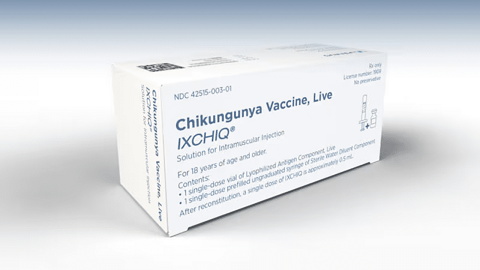
On May 9, 2025, the U.S. Food and Drug Administration (FDA) and the U.S. Centers for Disease Control and Prevention (CDC) recommended a temporary pause in the use of Ixchiq, a live virus vaccine designed to prevent chikungunya virus infection, in adults aged 60 years and older. The recommendation follows reports of serious post-marketing adverse […]
CDC’s ACIP Forms Workgroup to Back New Lyme Disease Vaccine and Monoclonal Antibody Products

At an Apr.15-16, 2025 meeting of the U.S. Centers for Disease and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP), it was announced that a workgroup had been formed to further development of a new vaccine for Lyme disease. The first session of the new group will take place in May with a presentation scheduled […]
Pfizer/BioNTech mRNA Combo Influenza and COVID Shot Efficacy Fails in Trial
Pfizer and BioNTech recently announced that a new mRNA vaccine designed to protect against both COVID-19 and influenza in a single shot faced a major setback after failing to achieve one of its primary goals in a late-stage trial. The “primary goal” missed in the late-stage trials was the ability to effectively prevent influenza B. […]
Novel mRNA Lyme Shots Could Be Released in 2025

Two novel messenger RNA (mRNA) shots for Lyme disease currently in late-stage clinical trials could hit the market as early as 2025, Moderna announced earlier this year. Also in the race to develop the first-ever mRNA product to prevent Lyme disease are Pfizer and Austria-based Valneva, which entered into a collaborative agreement in April 2020 […]
German Report Reveals 1 in 25 Insured Individuals Treated for COVID Shot Reactions
Techniker Krankenkasse, Germany’s largest health insurance company, reported that 437,593 of 11 million (1 in 25) insured individuals in 2021 had to undergo medical treatment as a result of adverse reactions from COVID-19 shots.1 COVID shots currently approved for use in Germany include Novavax’s Nuvaxovid, Moderna’s Spikevax, Pfizer/BioNTech’s Comirnaty, Johnson & Johnson/Janssen’s Ad26.COV2.S, AstraZeneca/Oxford University’s […]
Another Shot at a Vaccine for Lyme Disease
The U.S. National Institutes of Health (NIH) has provided the University of Maryland with a $3.5 million dollar grant to develop a vaccine for Lyme disease.1 Principal investigators Utal Pal, PhD, professor at the Virginia-Maryland College of Veterinary Medicine at the University of Maryland College of Agriculture and Natural Resources and Matthias Schnell, PhD, director […]
