NC Court Rules Federal PREP Act Protects Forced Vaccination Without Parental Consent
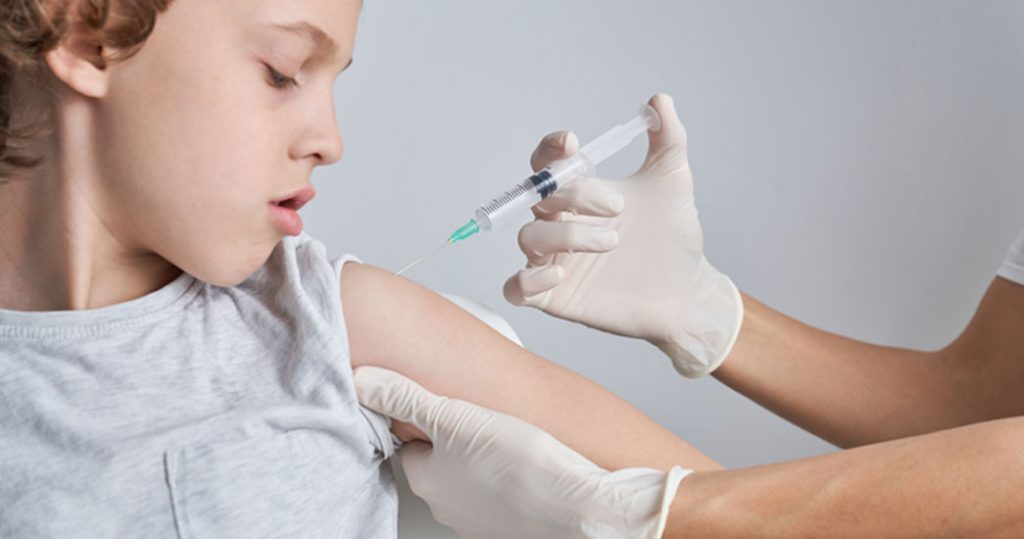
A North Carolina Court of Appeals found that a clinic, where personnel gave a 14-year-old boy a COVID-19 shot without his consent or parental consent, was protected by the Public Readiness and Emergency Preparedness Act (PREP Act). The court concluded that the Guilford Board of Education, which hosted the clinic, was also covered by the […]
Walgreens Sued for Wrongfully Giving COVID Shots to Children

Parents in Evansville, Indiana have filed a lawsuit against Walgreens pharmacy store for administering the wrong vaccine to their children. Alexandra and Joshua Price took their four-year-old son and five-year-old daughter to Walgreens to receive influenza vaccines on Oct. 4, 2021, but instead the children were given adult doses of Pfizer/BioNTech’s Comirnaty mRNA ((messenger ribonucleic […]
The First Amendment, Brought to You By Pfizer

Pfizer now claims the right of a corporate sovereign, arguing that states have “no legitimate interest in regulating” the company’s commercial speech while demanding the power to censor Americans’ newsfeeds. The call for pharmaceutical supremacy came in Pfizer’s response to Texas Attorney General Ken Paxton’s suit alleging that Pfizer committed fraud and “conspired to censor public discourse.” Pfizer embraces […]
Claims for Injury, Death from COVID Shots Surge

Opinion | The Countermeasures Injury Compensation Program (CICP) is a program of the U.S. government that provide financial compensation for people who have suffered serious injuries or death from vaccines, biologics, antivirals or other drugs, as well medical devices used to “diagnose, prevent or treat a declared pandemic, epidemic or security threat.” The program was […]
FDA Approves BioNTech’s Comirnaty. Pfizer COVID Shot Remains Experimental.
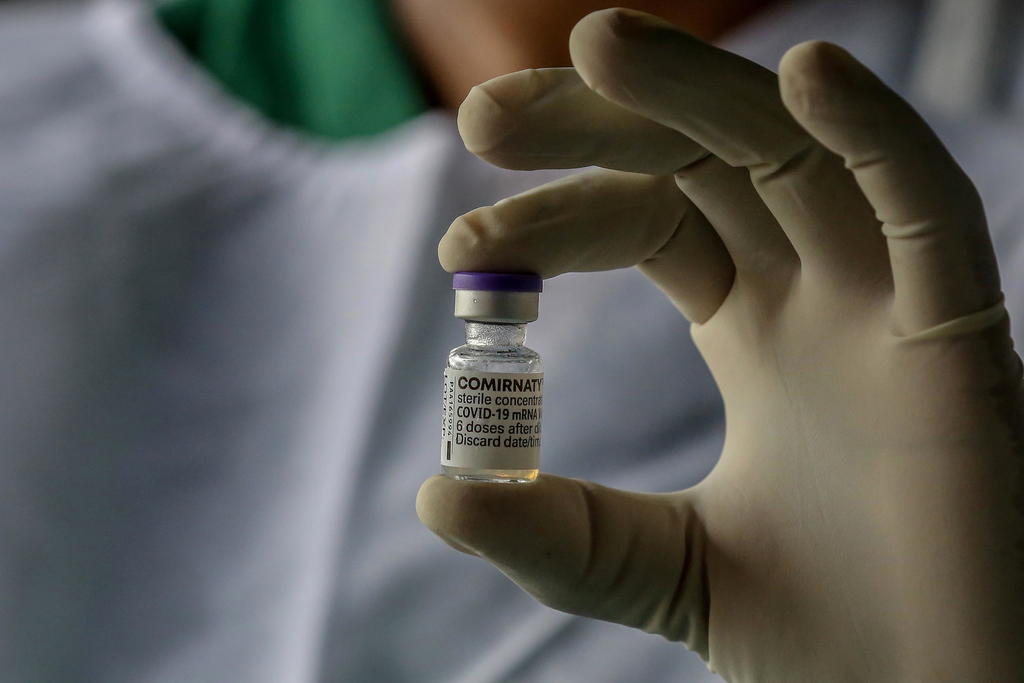
On Aug. 23, 2021, the U.S. Food and Drug Administration (FDA) gave full approval to a Biologics License Application (BLA) submitted by BioNTech Manufacturing GmbH of Mainz, Germany on May 18, 2021 for a biologic drug called COVID Vaccine, mRNA. The FDA gave permission to BioNTech to label the product “Comirnaty” and market it in […]
Mandating a Novel Vaccine is a Novel Legal Issue

The emergency use authorization (EUA) granted to vaccine manufacturers by the U.S. Food and Drug Administration (FDA) to allow experimental COVID-19 vaccines to be distributed to the U.S. population states that the product is “an investigational vaccine not licensed for any indication” and requires that all “promotional material relating to the COVID-19 Vaccine clearly and […]

