Comparing Risks: The Right and Wrong Way

Now, after three years with COVID-19, the pandemic is ebbing away worldwide. What’s still high, however, is the number of reports to the pharmaceutical authorities regarding serious symptoms and injuries after COVID vaccination. In Sweden, they’ve even continued to increase at a constant rate during the past year. Ever since the middle of 2021, I’ve tried […]
Pfizer COVID Shot Linked to Death of Woman in Minnesota
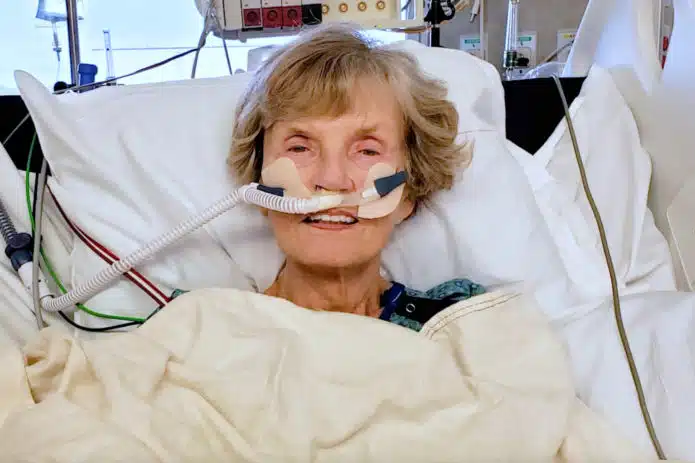
An 82-year-old woman died in a hospital in Twin Cities, Minnesota on June 2, 2022, 16 days after receiving a second booster dose of Pfizer/BioNTech’s Comirnaty messenger RNA (mRNA) COVID-19 shot. Described by one of her two daughters, Leanne Goth, as a “vital, energetic person,” Darlene Carlson was vaccinated on May 17 and within five […]
U.S. to Spend Billions on New COVID Vaccines Despite Failure to Prevent Infection
The Biden administration announced earlier this month that the U.S. government will spend at least $5 billion on Project NextGen to rapidly develop “next-generation” SARS-CoV-2 vaccines and treatments despite evidence that current COVID vaccines do not prevent infection and transmission of the new coronavirus.1 Following a similar approach to “Operation Warp Speed” initiated by the […]
Pfizer Hid Data on Waning Immunity

In late 2020, the airways became saturated with triumphant reporting of Pfizer and Moderna’s “95% effective” COVID-19 vaccines. Millions rolled up their sleeves with the belief that reaching herd immunity would end the pandemic. But by June 2021, the pandemic endgame story had gone off script. Highly vaccinated countries like Israel were experiencing a new wave of […]
Death of Teenage Girl in Japan Linked to Pfizer mRNA COVID Shot
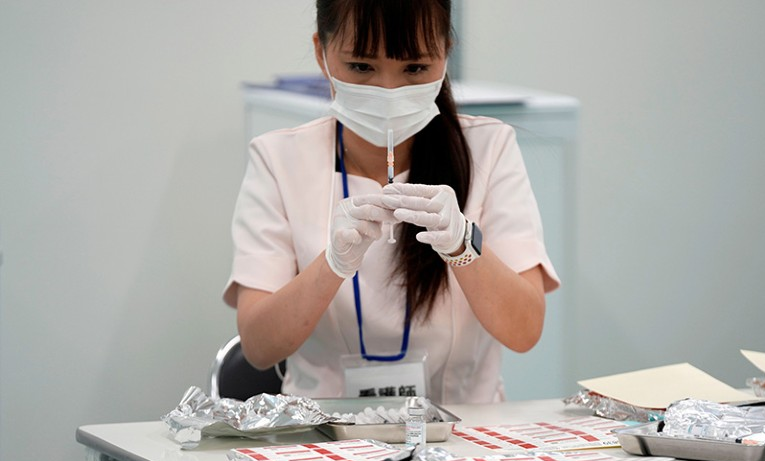
A 14-year-old girl died in Japan on Aug. 12, 2022, two days after receiving a third dose of Pfizer/BioNTech’s Comirnaty messenger RNA (mRNA) COVID-19 shot. The child reportedly developed a fever of 100.22 °F a day after the shot. That evening, she experienced breathing problems but still managed to fall asleep.1 2 3 According to […]
Global Pediatric Vaccines Market to Exceed $69 Billion by 2030

A report released by Coherent Market Insights showed that pediatric vaccines market will surpass $69.482 billion by 2030.1 The main pharmaceutical companies in the global market for pediatric vaccines include Bharat Biotech, Emergent BioSolutions, GSK, Indian Immunologicals, Merck, Panacea Biotec, Pfizer, Sanofi, Serum Institute and Zydus Cadila.2 Pediatric Vaccine Approvals by Governments Will Drive Vaccine […]
