NIH Begins Testing Mixed COVID-19 Vaccine Schedules

The U.S. National Institutes of Health (NIH) has announced that it has begun an early stage Phase 1/2 clinical trial to examining what happens when an adult who is fully vaccinated with one type of COVID-19 vaccine, is given a different booster vaccine from another manufacturer three to four months later.1 Currently, there are three […]
U.S. Government Partnering With Community Leaders to Increase COVID-19 Vaccine Uptake

The U.S. Department of Health and Human Services (HHS) has proposed an initiative called “The
Award Winning Investigative Journalist Provides Evidence of Origins of SARS-CoV-2

Although it has been suggested since the start of the pandemic in early 2020 that SARS-CoV-2, the new mutated coronavirus virus that causes COVID-19 disease, may have originated…
Moderna Expects $18.4 Billion in Sales from Its COVID-19 Vaccine This Year

Moderna, Inc. expects to generate $18.4 billion from sales of its experimental mRNA-1273 COVID-19 vaccine being distributed in the U.S. under an Emergency Use Authorization (EUA) granted by the Food and Drug Administration (FDA). This will be the first time that the U.S. based pharmaceutical company will make a profit since it was established in […]
Moderna, Pfizer Test mRNA Experimental Biologics on Children

On Dec. 10, 2020, American biotechnology company Moderna, Inc., which is pioneering the development of experimental messenger RNA (mRNA) therapeutics and vaccines, gave a 12-year old a dose of the company’s mRNA-1273 vaccine in a Phase 2/3 study of the new vaccine. Moderna CEO Stephane Bancel said, “Our goal is to generate data in the […]
Another Shot at a Vaccine for Lyme Disease
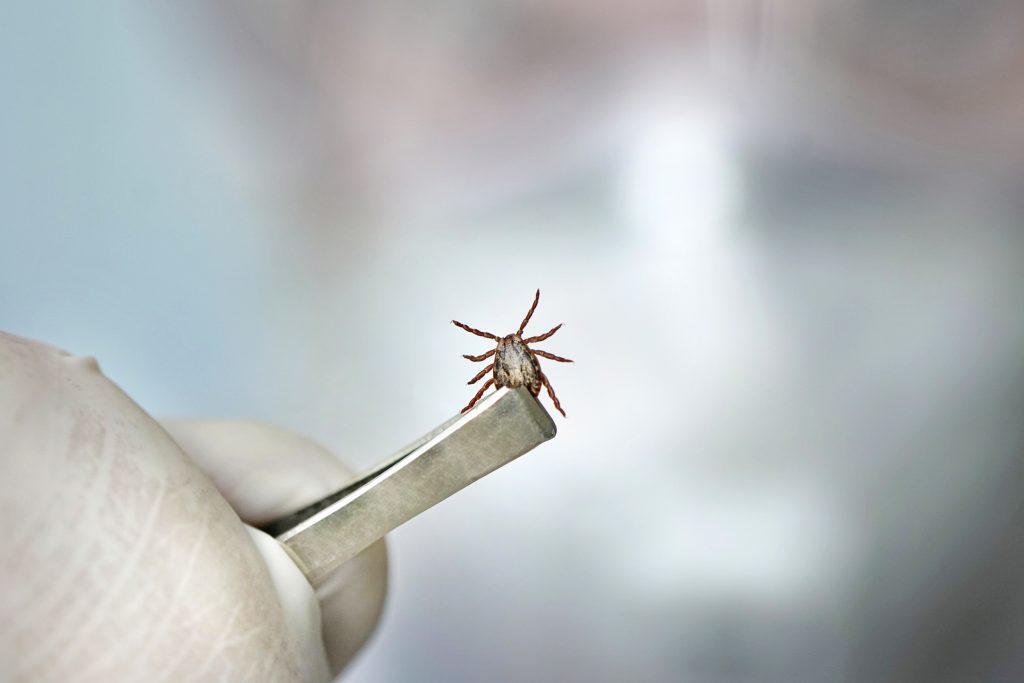
The U.S. National Institutes of Health (NIH) has provided the University of Maryland with a $3.5 million dollar grant to develop a vaccine for Lyme disease.1 Principal investigators Utal Pal, PhD, professor at the Virginia-Maryland College of Veterinary Medicine at the University of Maryland College of Agriculture and Natural Resources and Matthias Schnell, PhD, director […]
