FDA Licenses Merck’s Capvaxive Pneumococcal Vaccine
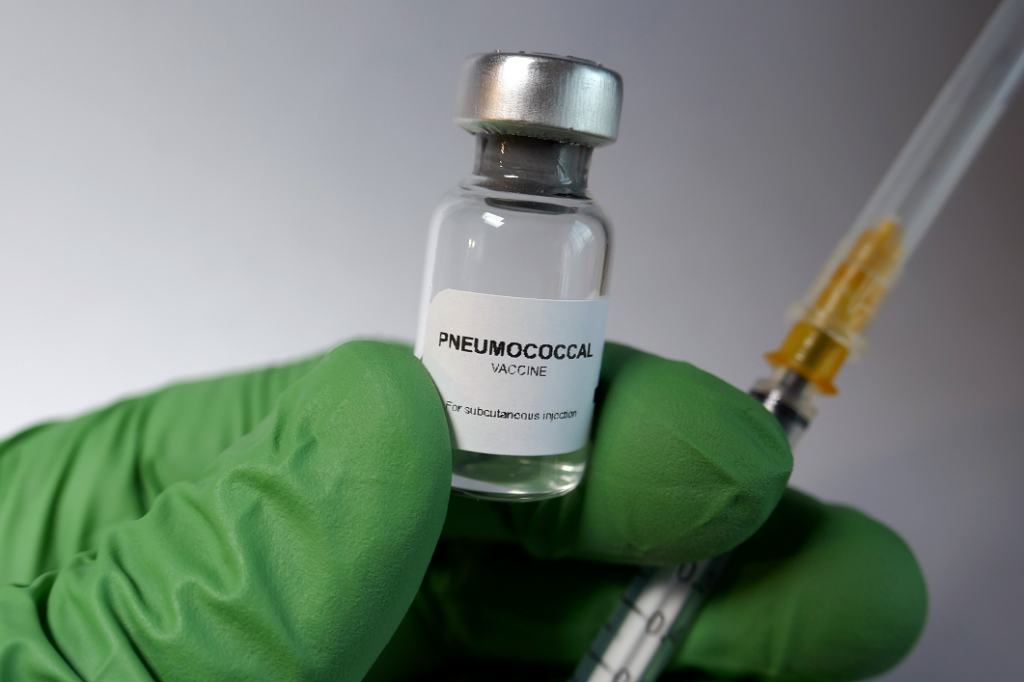
On June 17, 2024, the U.S. Food and Drug Administration (FDA) licensed Merck & Company, Inc.’s new pneumococcal conjugate vaccine Capvaxive (formerly V116) for adults 18 years of age and older. The vaccine is designed to protect against 21 types of the bacteria causing pneumococcal disease, which can lead to severe infection in the lungs […]
Survey Finds 50 Percent of Americans Will Not Get Influenza Vaccine
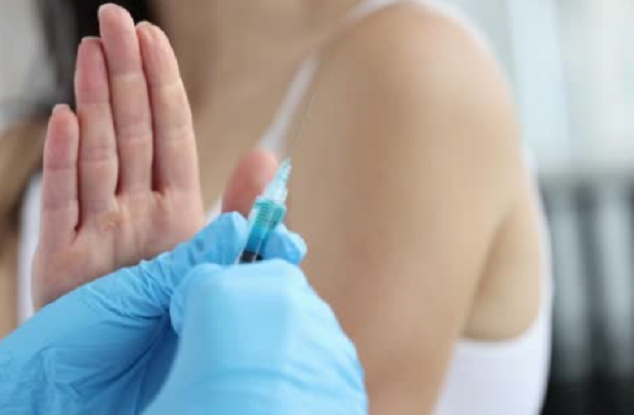
A recent survey, which was conducted by the National Foundation for Infectious Diseases (NFID) to better understand the public’s attitudes and beliefs about influenza, COVID-19, respiratory syncytial virus (RSV) and pneumococcal disease, found that many adults in the United States are not concerned about these respiratory diseases. As a result, many Americans do not plan […]
