FDA Approves MenQuadfi Meningococcal Vaccine for Six-Week-Old Infants
The U.S. Food and Drug Administration (FDA) has licensed Sanofi’ SA’s four-valent MenQuadfi meningococcal vaccine for use in infants aged six weeks to 23 months old to prevent invasive meningococcal disease. Previously approved for children aged two and older, MenQuadfi is now the first meningococcal vaccine included on the U.S. Centers for Disease Control and […]
Young Woman With Blood Disorder Develops Painful Severe Reaction to Multiple Vaccinations
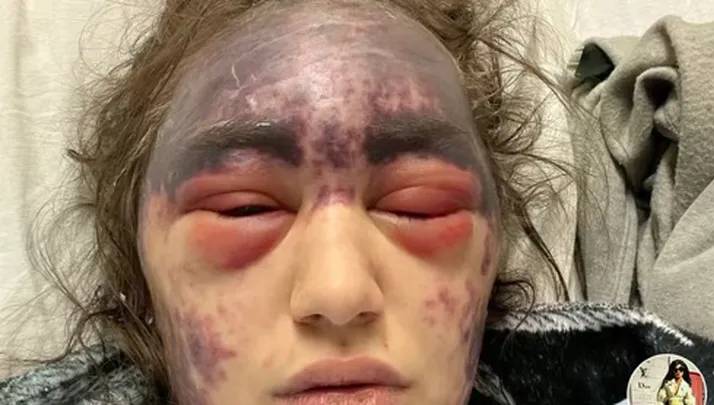
A 23-year-old woman being treated for a rare autoimmune blood disorder at UCI Medical Center in Orange, California earlier this month report suffered a severe reaction after receiving multiple vaccines for tetanus, pneumococcal and meningitis at the hospital. Alexis Lorenze, who in January 2024 was diagnosed with Paroxysmal Nocturnal Hemoglobinuria (PNH), went to UCI Medical […]
AstraZeneca Halts, Resumes COVID-19 Vaccine Trial After Serious Neurological Event
News update, Sept. 15, 2020—U.S. regulators paused AstraZeneca’s vaccine trial in the U.S. while the National Institutes of Health (NIH) launches an investigation of its own into a serious side effect in an unnamed patient in Britain. “The highest levels of NIH are very concerned,” said Dr. Avindra Nath, intramural clinical director and a leader […]
