FDA Revokes EUA for Johnson & Johnson/Janssen’s COVID Vaccine
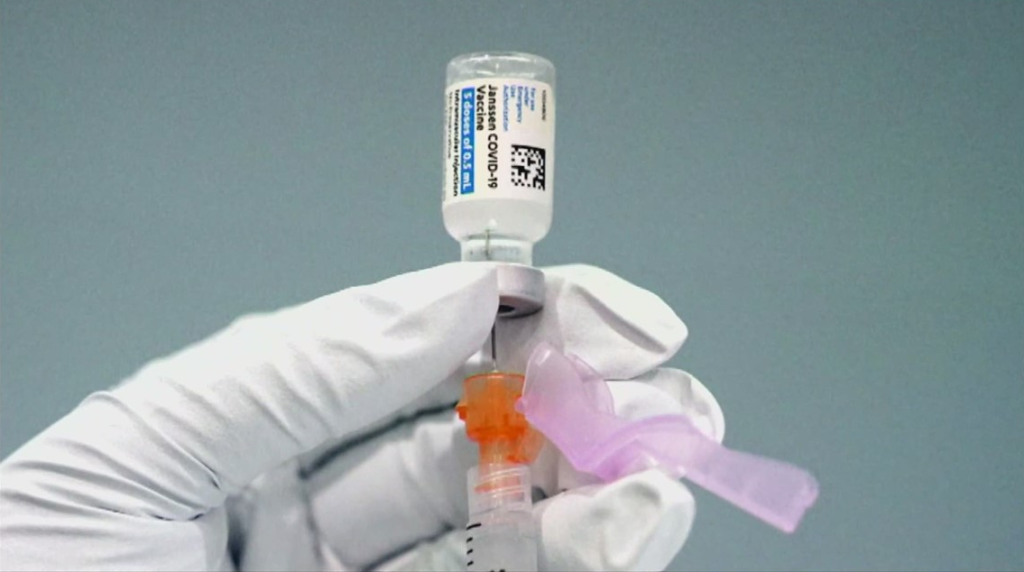
On June 1, 2023, the U.S. Food and Drug Administration (FDA) withdrew the Emergency Use Authorization (EUA) Johnson & Johnson/Janssen’s adenovirus vectored Ad26.COV2.S (also known as JNJ-78436735) COVID-19 vaccine that had been granted to the companies on Feb. 27, 2021. The FDA noted that Janssen requested the voluntary withdrawal of the EUA in a letter […]

