Unknown Risks of COVID Shot Harm Revealed in New Report

At the request of the Health Resources Services Administration (HRSA), an agency in the U.S. Department of Health and Human Services (DHHS), the National Academies of Science, Engineering and Medicine assembled a committee of physicians and scientists to conduct a review of the evidence that COVID-19 shots can cause serious harm to health. The evidence […]
Pfizer COVID Shot Linked to Death of Woman in Minnesota
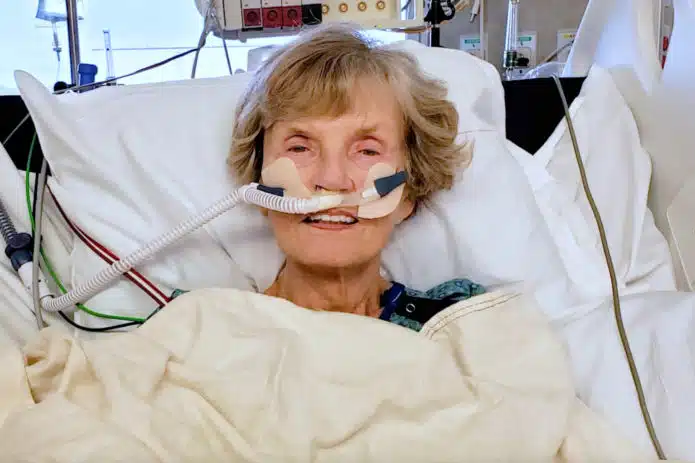
An 82-year-old woman died in a hospital in Twin Cities, Minnesota on June 2, 2022, 16 days after receiving a second booster dose of Pfizer/BioNTech’s Comirnaty messenger RNA (mRNA) COVID-19 shot. Described by one of her two daughters, Leanne Goth, as a “vital, energetic person,” Darlene Carlson was vaccinated on May 17 and within five […]
Coronavirus Testing Lab Defrauded U.S. Government of $83 Million

The U.S. Department of Justice (DOJ) recently announced criminal charges against Zishan Alvia, owner of Laboratory Elite, a SARS-CoV-2 virus testing laboratory in Chicago accused of a scheme in which Alvia obtained $83 million by fraudulently billing the U.S. Health Resources and Services Administration’s (HRSA) Uninsured Program for reimbursement for SARS-CoV-2 tests.1 HRSA’s Uninsured Program […]
U.S. Facing Severe Shortage of Infectious Disease Physicians

In light of the COVID-19 pandemic, there has been a focus on the shortage of doctors opting to specialize in infectious disease. According to forecasts by the U.S. Health Resources and Services Administration (HRSA), the United States will face a serious shortage of infectious disease doctors with estimates showing that that by 2035 there will […]
COVID-19 Vaccine Injury Compensation Program Described as Legal Black Hole

Attorneys familiar with vaccine injury claims have described the current compensation program for COVID-19 vaccines as a legal “black hole” and inadequate for addressing the vaccine injury claims associated with the fast-tracked COVID vaccines.1 Attorney Altom Maglio says he has been contacted by hundreds of people who suspect they have suffered COVID vaccine-related injuries but […]
Claims for Injury, Death from COVID Shots Surge

Opinion | The Countermeasures Injury Compensation Program (CICP) is a program of the U.S. government that provide financial compensation for people who have suffered serious injuries or death from vaccines, biologics, antivirals or other drugs, as well medical devices used to “diagnose, prevent or treat a declared pandemic, epidemic or security threat.” The program was […]

