ACIP Updates Influenza Vaccine Guidelines for Seniors

The Advisory Committee on Immunization Practices (ACIP) for the U.S. Centers for Disease Control and Prevention (CDC) has issued new updates for influenza vaccines for 2020–2021 “flu season,” primarily for Fluzone and Fluad vaccines. In November 2019, U.S. Food and Drug Administration (FDA) licensed inactivated injectable Fluzone High-Dose Quadrivalent (HD-IIV4) for use in persons aged […]
Fluzone Quadrivalent Influenza Vaccine Approved by FDA for Elderly
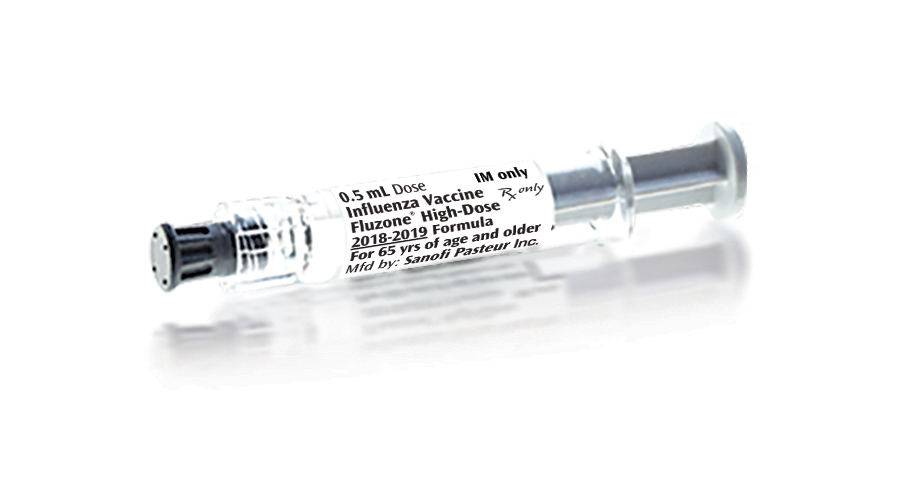
The U.S. Food and Drug Administration (FDA) has approved a supplemental Biologics License Application (BLA) for the Fluzone high-dose quadrivalent influenza vaccine for use by adults 65 years of age and older. Fluzone is manufactured by Sanofi Pasteur, Inc. of France.1 2 The Fluzone high-dose influenza vaccine was approved by the FDA in 2009 as […]
