The Dramatic Degradation of the Human Immune System

In many countries worldwide over the past three and a half years the health and financial situation of the population has been falling downhill in a way that has never been seen in the past decades. Trend analysis shows increasing numbers of excess mortalities in all ages and a steep rise in a decay of […]
Popular Weight-Loss Drugs Linked to Stomach Paralysis, Suicide and More

Just two months after the European Medicines Agency (EMA) issued a safety signal citing concerns about a possible link to all types of thyroid cancer in people using Ozempic, doctors are sounding the alarm on even more dangerous health risks associated with the popular weight loss and diabetes medication. Negative health outcomes reportedly associated with […]
Ozempic Weight Loss Drug Linked to Increased Risk of Thyroid Cancers
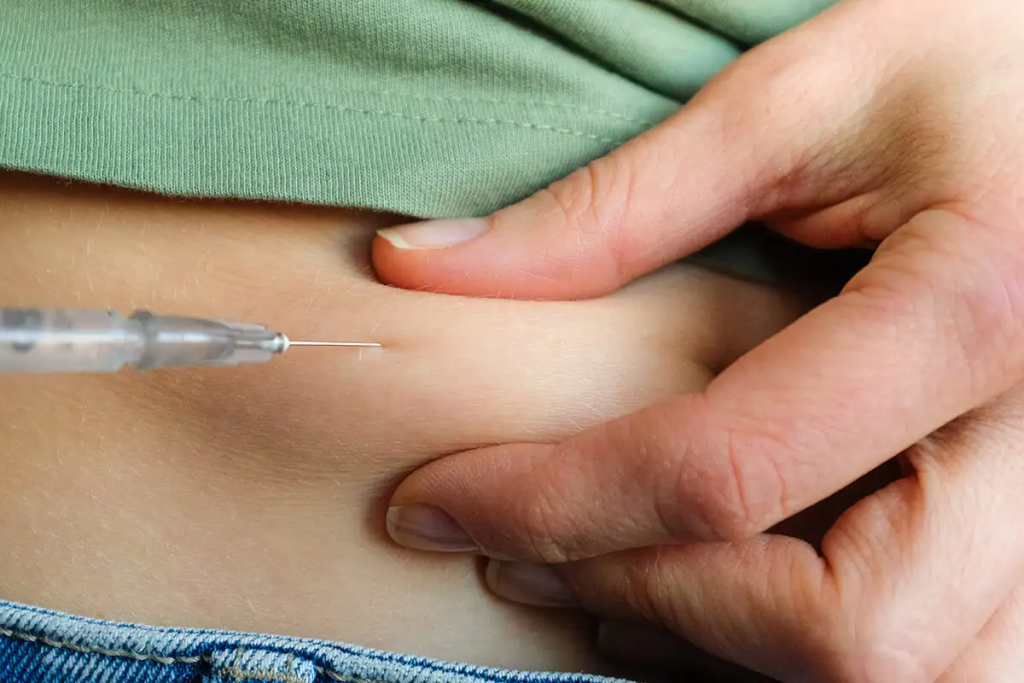
New weight loss drug Ozempic has taken the world’s obesity epidemic by storm, but like most weight loss drugs that precede it over the past century, shedding extra pounds may not be the only outcome for consumers: Last month, the European Medicines Agency (EMA), a European Union (E.U.) agency that oversees and evaluates pharmaceutical medications, […]
CDC, FDA Find Possible Link Between Pfizer’s Bivalent COVID Shot and Strokes

The U.S. Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) said last week that they are investigating a possible link between Pfizer/BioNTech’s bivalent messenger RNA (mRNA) Comirnaty COVID biologic and an increased risk of a type of ischemic stroke among people 65 years of age and older. Data from the […]
COVID Vaccines Have Saved Millions of Lives… in Models

As more and more questions are being asked by more and more scientists, health professionals and journalists, the narrative of “safe and effective” COVID-19 vaccines is crumbling by the day, and scientific truth is slowly beginning to impose itself. The simple scientific truth is that these vaccines are clinically useless, but not entirely harmless (no medical intervention is). […]
U.K. Coronor Says AstraZeneca’s Covid Vaccine Caused ‘Fit and Healthy’ Young Man to Die from Blood Clot in Brain

A 27-year-old man in England developed a blood clot in the brain and died on Apr. 20, 2021, three weeks after receiving the first dose of AstraZeneca/Oxford University’s experimental viral vectored Covid-19 vaccine Vaxzevria (also known as AZD1222), and a U.K. coroner determined his death was causally related to the vaccination. Jack Last of Stowmarket […]

