Moderna, Novavax Developing Combined Single-Dose COVID-19 and Influenza Vaccine
American pharmaceutical and biotechnology company Moderna, Inc., which partnered with the U.S. National Institutes of Allergy and Infectious Diseases (NIAID) to develop an mRNA COVID-19 vaccine being distributed in the United States under an Emergency Use Authorization (EUA) announced that it is developing a combined single-dose booster vaccine for COVID-19 and seasonal influenza.1 In a […]
FDA’s Expert Panel Recommends COVID-19 Booster Dose for Seniors and Others, Rejects Booster Dose for Children

Despite not convening the Vaccines and Related Biologic Products Advisory Committee (VRBPAC) last month to vote on effectiveness and safety of the Pfizer-BioNTech COVID-19 vaccine (licensed under the name COMIRNATY), the U.S. Food and Drug Administration (FDA) convened the advisory committee on Friday, Sept. 17, 2021 to vote on booster doses of the vaccine.1 The […]
The FDA Has a History of Rushed Drug Approvals

Two weeks ago, the Pfizer COVID-19 vaccine was reported as the first COVID-19 vaccine to receive full approval from the U.S. Food and Drug Administration (FDA) for individuals aged 16 and older.1 But, for some, questions remain regarding conflicting discrepancies of the wording surrounding the approval.2 While the FDA’s report states that full approval was […]
FDA Approves BioNTech’s Comirnaty. Pfizer COVID Shot Remains Experimental.
On Aug. 23, 2021, the U.S. Food and Drug Administration (FDA) gave full approval to a Biologics License Application (BLA) submitted by BioNTech Manufacturing GmbH of Mainz, Germany on May 18, 2021 for a biologic drug called COVID Vaccine, mRNA. The FDA gave permission to BioNTech to label the product “Comirnaty” and market it in […]
Canada Adds Bell’s Palsy Warning to Pfizer COVID Shot

Health Canada, the Canadian government’s department responsible for national health policy, earlier this month updated the product information label for Pfizer/BioNTech’s experimental messenger RNA (mRNA) biologic (also known as BNT162b2 or “Comirnaty” vaccine) to reflect reported cases of Bell’s palsy—an immune mediated neurological disorder characterized by temporary muscle weakness or paralysis on one side of […]
Women Experiencing Enlarged Breast Lymph Nodes After COVID-19 Vaccination
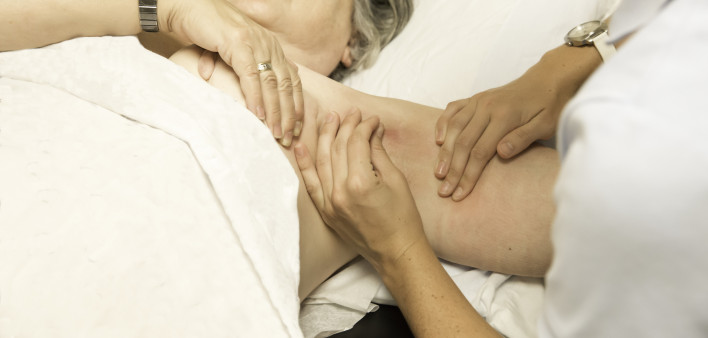
Soon after the United States began rolling out experimental COVID-19 vaccines distributed under an Emergency Use Authorization (EUA), doctors began seeing an influx of female patients making mammogram appointments after the women experienced enlarged lymph nodes, known as axillary lymphadenopathy, in the armpit area after receiving a dose of mRNA COVID-19 vaccine.1 Laura Esserman, MD, […]
