Study Finds Increased Risk of Stroke in Elderly After COVID and High Dose/Adjuvanted Influenza Shots

On Jan. 13 2023, the U.S. Food and Drug Administration (FDA) and the U.S. Centers for U.S. Disease Control and Prevention (CDC)) issued a public statement regarding the identification of a preliminary safety signal for ischemic stroke among adults over the age of 65 that occurs between one to 21 days after receiving Pfizer/BioNTech’s monovalent […]
Pfizer Will Charge Patients $1,390 for COVID Anti-Viral Paxlovid

Pfizer, Inc. plans to charge consumers more than double the price charged to the U.S. government for its COVID-19 anti-viral drug nirmatrelvir-ritonavir, sold under the brand name Paxlovid. A five-day course of the drug to treat COVID will soon cost patients $1,390. According to researchers, it costs Pfizer $13 to manufacture the supply of pills […]
CDC Says Overweight People Need Longer Needles for Vaccinations
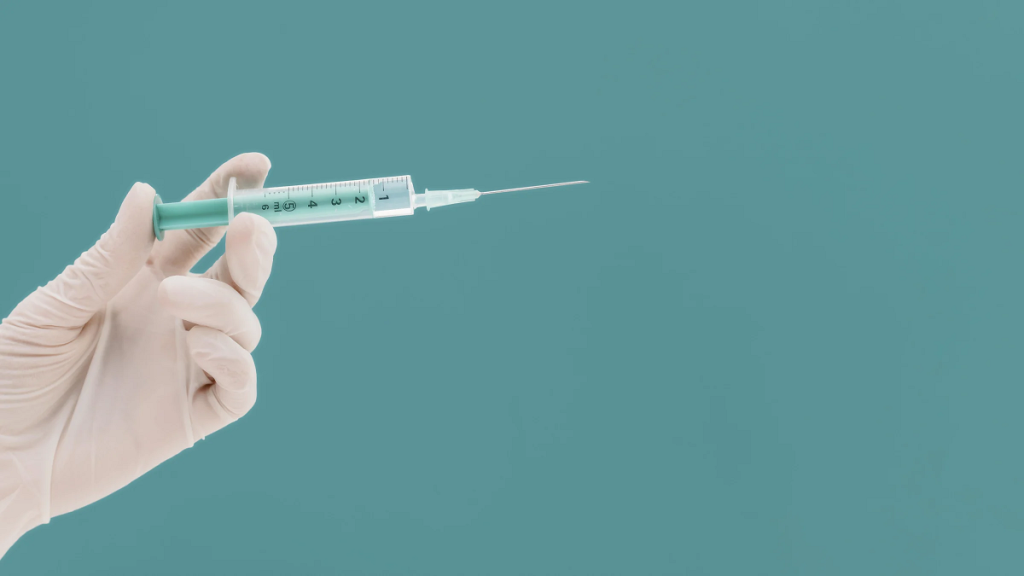
If you were among the many who experienced a breakthrough SARS-CoV-2 infection despite getting a COVID-19 shot (or two or three), federal health officials suggest that—if you are overweight or obese—the length of the needle used to inject you might be to blame, not vaccine failure. New recommendations from the U.S. Centers for Disease Control […]
Nobel Prize Goes to Dubious mRNA Research Used to Develop COVID Shots

On Oct. 2, 2023, the Nobel Assembly at Karolinska Institutet in Stockholm, Sweden awarded the 2023 Nobel Prize in Physiology or Medicine jointly to scientists Katalin Karikó, PhD and Drew Weissman, MD, PhD “for their discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19.” The mRNA (messenger RNA) “vaccines”—which […]
Study Links COVID Shots to Unusual Vaginal Bleeding in Women

A few years after reports began to surface of women around the world experiencing menstrual changes after being vaccinated for COVID-19, the Pfizer/BioNTech, Moderna/NIAID and AstraZeneca/Oxford University COVID shots have been officially linked to unexplained vaginal bleeding—even in women without regular periods—according to a new study published in Science Advances last week. The large cohort […]
Pulling Out All the Stops to Sell More COVID Shots

This year, Pfizer, Inc. and Moderna, Inc. have experienced sharply declining sales of their messenger RNA (mRNA) COVID-19 shots, Comirnaty and Spikevax. Pfizer reported first-quarter (ending Mar. 31, 2023) sales of $3 billion for Comirnaty—down 75 percent from the same period in 2022. It posted second-quarter (ending June 30) sales of $1.49 billion—down 83 percent […]

