WHO Prequalifies Takeda’s Qdenga Dengue Vaccine
On May 10, 2024, the World Health Organization (WHO) granted prequalification status to the Qdenga (TAK-003) dengue vaccine, developed by Takeda Pharmaceutical Company Ltd. of Tokyo, Japan. The live attenuated virus vaccine is recommended to be given in two doses three months apart to children six to 16 years old living in areas where dengue […]
The Impending U.S. ICD Vaccine Passport and Its Unconstitutionality

The CDC recently codified International Classification of Disease (ICD) codes for COVID-19 vaccine status. ICD codes are extensively used in medical records, medical insurance data and health research to classify precisely disease states as well as injuries from exogenous agents such as accidents, medication and medical device injuries, toxic chemicals, etc. Vaccination status is not […]
Fluzone Quadrivalent Influenza Vaccine Approved by FDA for Elderly
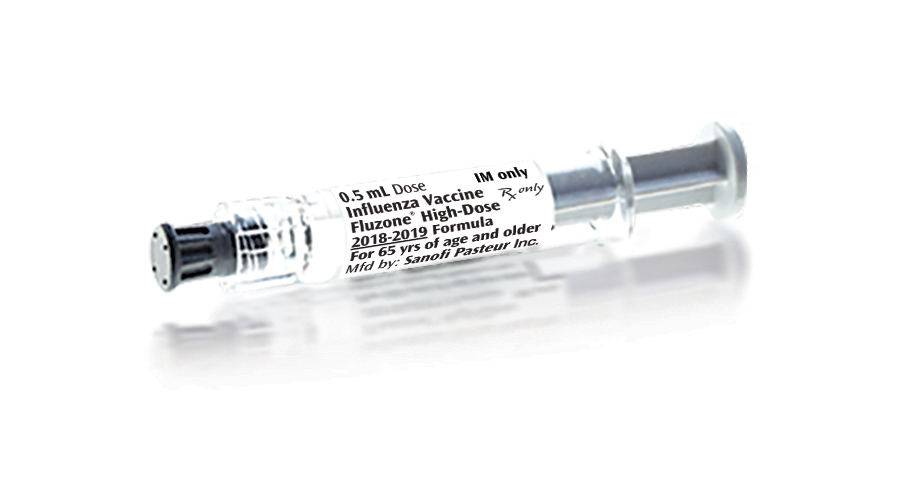
The U.S. Food and Drug Administration (FDA) has approved a supplemental Biologics License Application (BLA) for the Fluzone high-dose quadrivalent influenza vaccine for use by adults 65 years of age and older. Fluzone is manufactured by Sanofi Pasteur, Inc. of France.1 2 The Fluzone high-dose influenza vaccine was approved by the FDA in 2009 as […]
Novartis Manipulated Data in FDA Biologics License Application

On Aug. 6, 2019, the U.S. Food and Drug Administration (FDA) issued a statement saying that Swiss pharmaceutical company Novartis AG submitted a biologics license application (BLA) for the spinal muscular atrophy (SMA) gene therapy Zolgensma (onasemnogene abeparvovec-xioi) with manipulated data and failed to inform regulators until June 28, 2019—more than a month after the […]
Merck Plans to Up Production of Experimental Ebola Vaccine
In response to the current outbreak of Ebola in the Democratic Republic of the Congo (DRC) which began in August 2018, the World Health Organization (WHO) has declared the outbreak a Public Health Emergency of International Concern (PHEIC). There have been about 2,500 confirmed cases of Ebola in the DRC and at least 1,737 people have […]

