U.K. Coronor Says AstraZeneca’s Covid Vaccine Caused ‘Fit and Healthy’ Young Man to Die from Blood Clot in Brain

A 27-year-old man in England developed a blood clot in the brain and died on Apr. 20, 2021, three weeks after receiving the first dose of AstraZeneca/Oxford University’s experimental viral vectored Covid-19 vaccine Vaxzevria (also known as AZD1222), and a U.K. coroner determined his death was causally related to the vaccination. Jack Last of Stowmarket […]
New Autopsy Report Reveals Those Who Died Suddenly Were Likely Killed by the COVID Vaccine

A major new autopsy report has found that three people who died unexpectedly at home with no pre-existing disease shortly after COVID vaccination were likely killed by the vaccine. A further two deaths were found to be possibly due to the vaccine. The report, published in Clinical Research in Cardiology, the official journal of the German Cardiac […]
Thyroid Disorders Can Be Caused or Worsened by COVID Shots
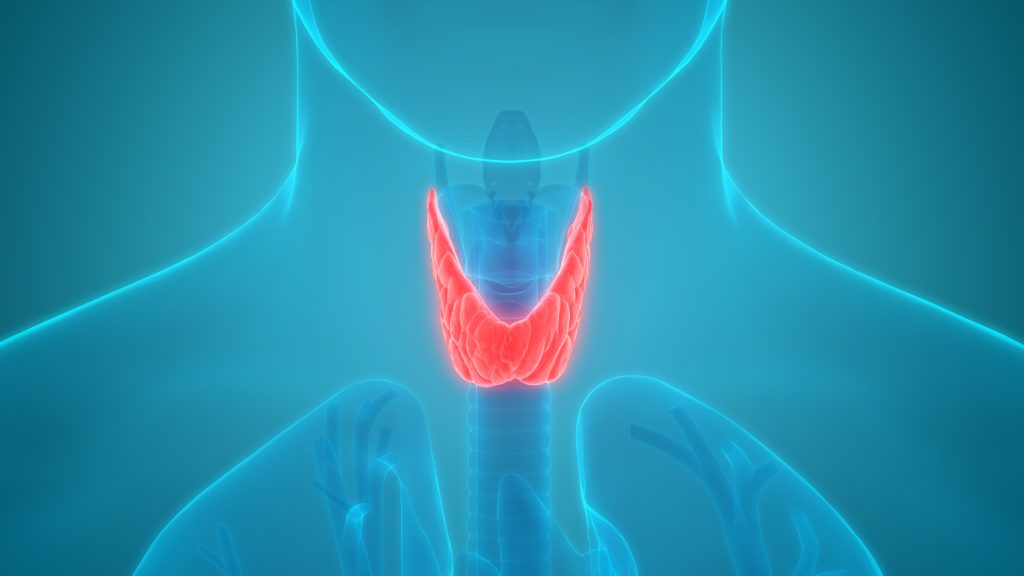
There have been a number of reports published in the medical literature describing cases of thyroid autoimmunity developing in individuals, who have received different types of COVID-19 shots.1 Cases occur in individuals who have never been diagnosed with a thyroid disorder and in those whose thyroid disorders worsen after COVID vaccinations. The thyroid is an […]
More Than 200 Million Doses of COVID Shots Dumped Due to Diminishing Demand
Nearly two years after the introduction of COVID-19 biologics around the world, the pharmaceutical industry is facing a waning demand for these products. Scott Rosenstein, a New York-based health care adviser to Eurasia Group said: Supply is exceeding demand in much of the world, even with many countries rolling out booster shots. Once the perception set […]
Another Study Confirms COVID Vaccine Alters Menstrual Cycles

A study published in the BMJ Medicine in August 2022 involving almost 20,000 women found an association between those who received a COVID-19 shot and an increased length in menstrual cycles. Of the 15,000 vaccinated women in the study, two-thirds received Pfizer/BioNTech’s COVID messenger RNA (mRNA) biologic, but the data also included women who received […]
Organ Transplant Failures After COVID Vaccination
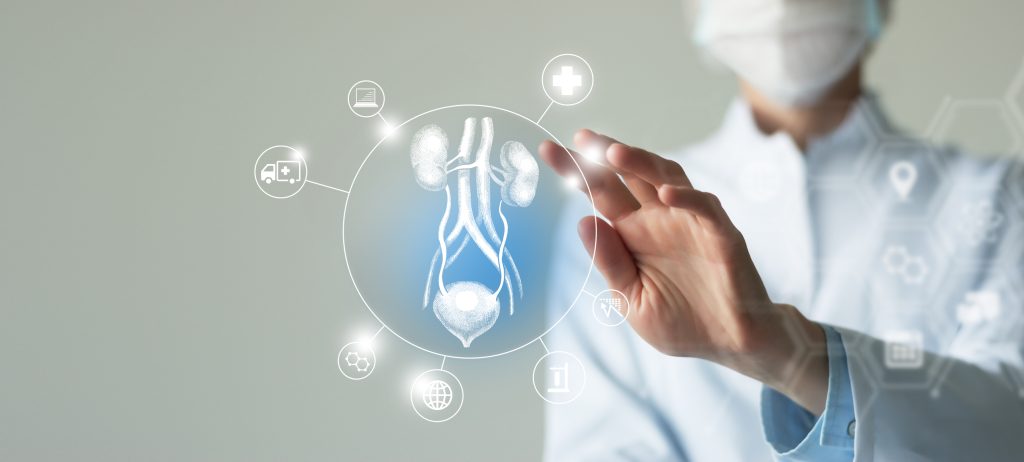
In 2022, researchers have published articles in the medical literature reporting organ transplant failures in people who have recently received COVID-19 shots. These reports include corneal graft rejections and solid organ transplant failures involving the kidneys, livers, lungs, hearts and pancreas. A study by Japanese researchers published in June in the Journal of Clinical Medicine […]
